A Durable Option for Complex AIOD (Aortoiliac Occlusive Disease)
Join the next generation of care with the GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis (VBX Stent Graft)
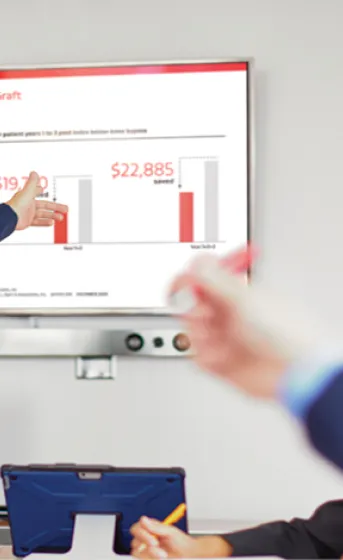
5-year VBX Stent Graft Follow-up
Decide with data
Previously, the Gore VBX FLEX Clinical Study 3-year follow-up found the VBX Stent Graft to be a robust and durable treatment option for AIOD.1 Now, sustained patient benefit and durability are demonstrated through 5 years.
Investigator Insights
Andrew Holden, M.D. discusses the background, outcomes and implications of this 5-year long-term data.
Physician-initiated 5-year VBX Stent Graft follow-up
Objective and methodology2
- This physician-initiated study enrolled 59 patients from 3 participating centers that were representative of the VBX FLEX Study 3-year follow-up cohort. Twenty-eight patients completed the 5-year follow-up.
- The primary durability endpoint was long-term primary patency.
DURABLE CLINICAL OUTCOMES THROUGH 5 YEARS2:
89.5%
primary patency per lesion
96.1%
primary assisted patency per lesion
89.1%
feedom from target lesion revascularization per subject
SUSTAINED PATIENT BENEFIT THROUGH 5 YEARS2:
100%
of patients improved ≥ 1 Rutherford category from baseline
.15
improvement in mean resting ankle-brachial index (ABI)
(P < .001, .95 mean ABI)
3x
improvement in median WIQ measures
Interested in more insights?
Hear more physician perspectives on the 5-year VBX Stent Graft follow-up
The case for long-term durable clinical outcomes:
Visit the VBX Stent Graft product page. >
NOW 6 Fr compatible
GREATER VERSATILITY
GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface*,†
Proven patency with unmatched versatility‡

Visit the VIABAHN® Device product page. >
VIABAHN® Device outcomes in iliac occlusive disease
Prospective, multicenter, single-arm study
53 patients (61 limbs) with iliac artery occlusion or stenosis (Mean lesion length: 6.9 cm; 48% of limbs in the external iliac artery)
91%
primary patency at 1 year3
95%
secondary patency at 1 year3
* As used by Gore, Heparin Bioactive Surface refers to Gore's proprietary CBAS® Heparin Surface.
† Also referred to as the GORE® VIABAHN® Endoprosthesis with PROPATEN Bioactive Surface in some regions.
‡ Across indications and configurations of covered stents.
1. Panneton JM, Bismuth J, Gray BH, Holden A. Three-year follow-up of patients with iliac occlusive disease treated with the Viabahn Balloon-Expandable Endoprosthesis. Journal of Endovascular Therapy 2020;27(5):728-736.
2. Holden A, Takele E, Hill A, et al. Long-term follow-up of subjects with iliac occlusive disease treated with the Viabahn VBX Balloon-Expandable Endoprosthesis. Journal of Endovascular Therapy. In press.
3. Lammer J, Dake MD, Bleyn J, et al. Peripheral arterial obstruction: prospective study of treatment with a transluminally placed self-expanding stent graft. Radiology 2000;217(1):95-104.

Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. RXOnly
INDICATIONS FOR USE IN THE U.S.: The GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis is indicated for the treatment of de novo or restenotic lesions found in iliac arteries with reference vessel diameters ranging from 5 mm–13 mm and lesion lengths up to 110 mm, including lesions at the aortic bifurcation. The GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis is also indicated for use with thoracoabdominal and pararenal branched devices indicated with the GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis as a branch component.§
CONTRAINDICATIONS: Do not use the GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis in patients with known hypersensitivity to heparin, including those patients who have had a previous incident of Heparin-Induced Thrombocytopenia (HIT) type II.
§ Not applicable to Reduced Profile GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis. (BXB catalogue numbers).
INDICATIONS FOR USE IN THE U.S.: The GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface is indicated for improving blood flow in patients with symptomatic peripheral arterial disease in superficial femoral artery de novo and restenotic lesions up to 270 mm in length with reference vessel diameters ranging from 4.0 – 7.5 mm. The GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface is indicated for improving blood flow in patients with symptomatic peripheral arterial disease in superficial femoral artery in-stent restenotic lesions up to 270 mm in length with reference vessel diameters ranging from 4.0 – 6.5 mm. The GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface is indicated for improving blood flow in patients with symptomatic peripheral arterial disease in iliac artery lesions up to 80 mm in length with reference vessel diameters ranging from 4.0 – 12 mm. The GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface is also indicated for the treatment of stenosis or thrombotic occlusion at the venous anastomosis of synthetic arteriovenous (AV) access grafts.
CONTRAINDICATIONS: The GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface is contraindicated for non-compliant lesions where full expansion of an angioplasty balloon catheter was not achieved during pre-dilatation, or where lesions cannot be dilated sufficiently to allow passage of the delivery system. Do not use the GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface in patients with known hypersensitivity to heparin, including those patients who have had a previous incident of Heparin-Induced Thrombocytopenia (HIT) type II.
The GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis is not authorized for the use with thoracoabdominal and pararenal branched devices indicated with the GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis as a branch component in Canada.