GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis Case Studies: Restoring flow in a patient with severe claudication and focal iliac calcification
Case submitted by Chris Metzger, M.D.
Kingsport, Tennessee
Challenge
- A 57-year-old male with severe intermittent claudication (Rutherford category 3).
- TASC II B lesion in the right common iliac artery (CIA) and focal iliac calcification.
- Relevant patient history:
- Current smoker with hypertension, hyperlipidemia and previous diagnosis of peripheral artery disease in the right limb that was treated with medication.
- Baseline ankle-brachial index (ABI) = 0.76.
Procedure
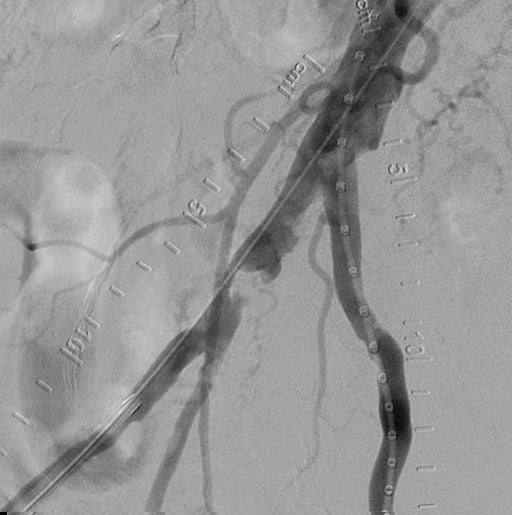
- Started with bilateral access, identified focal lesion in the distal right CIA that was ~80% stenosed.
- Angiography showed robust multi-vessel distal runoff down to the foot.
- Predilated with a 5 × 20 mm ABBOTT® ARMADA PTA Balloon, observed presence of calcification nodule that prevented full dilatation of the percutaneous transluminal angioplasty (PTA) balloon.
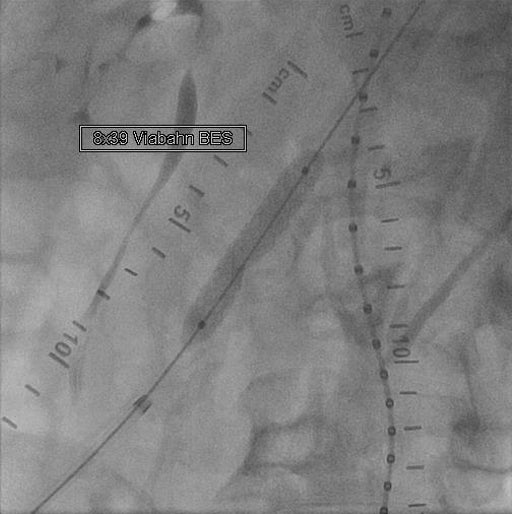
- Delivered and deployed an 8 mm × 39 mm GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis (VBX Stent Graft).
- Slight residual stenosis observed following VBX Stent Graft deployment.
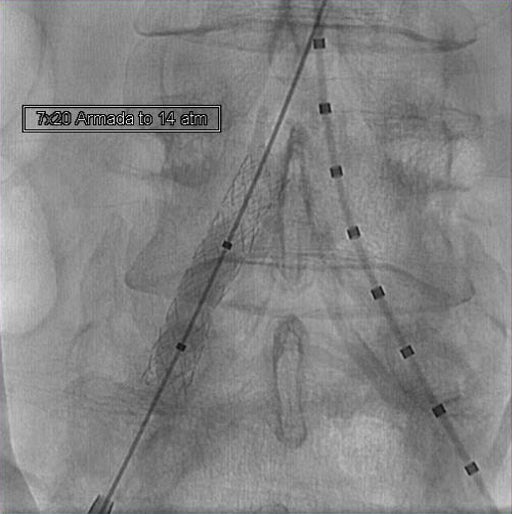
- Post-dilatation performed using a 7 × 20 mm ABBOTT® ARMADA PTA Balloon inflated to 14 atm (near rated burst pressure).
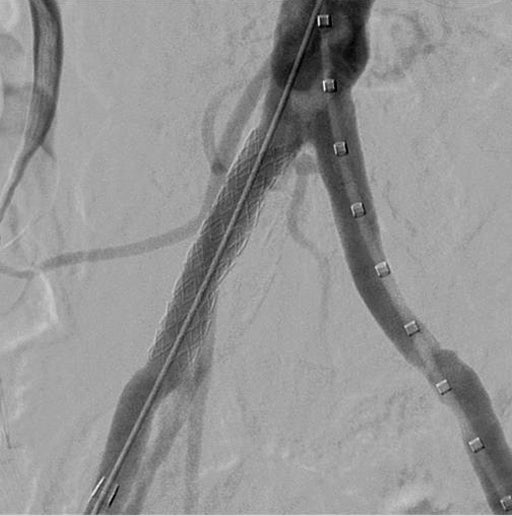
- Final angiogram showed complete restoration of patency in the right CIA with good distal outflow.
Case Takeaways
Calcified lesions present challenges in endovascular treatment of aortoiliac occlusive disease, including an increased risk of rupture. The VBX Stent Graft provides a barrier that mitigates concerns about neointimal hyperplasia and provides high radial strength to restore patency in even the most calcified lesions.1
- Bismuth J, Gray BH, Holden A, Metzger C, Panneton J; VBX FLEX Study Investigators. Pivotal study of a next-generation balloon-expandable stent-graft for treatment of iliac occlusive disease. Journal of Endovascular Therapy 2017;24(5):629-637.
Procedural outcomes based on usage of legacy GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis. (BXA catalogue numbers).
The outcomes and observations reported are based on individual case experience and the patients treated. The steps described here may not be complete, and are not intended to be a replacement for the Instructions for Use or the education, training and professional judgment of health care providers (HCP). HCPs remain solely responsible for making decisions about patient care and the use of medical technologies.
ABBOTT and ARMADA are trademarks of Abbott Vascular.

Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. RXOnly
INDICATIONS FOR USE IN THE U.S.: The GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis is indicated for the treatment of de novo or restenotic lesions found in iliac arteries with reference vessel diameters ranging from 5 mm–13 mm and lesion lengths up to 110 mm, including lesions at the aortic bifurcation. The GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis is also indicated for use with thoracoabdominal and pararenal branched devices indicated with the GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis as a branch component.*
CONTRAINDICATIONS: Do not use the GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis in patients with known hypersensitivity to heparin, including those patients who have had a previous incident of Heparin-Induced Thrombocytopenia (HIT) type II.
* Not applicable to Reduced Profile GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis. (BXB catalogue numbers).
The GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis is not authorized for use with thoracoabdominal and pararenal branched devices indicated with the GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis as a branch component in Canada.