GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis Case Studies: Restoring flow to a patient with stenosis at the aortoiliac bifurcation
Case submitted by Masahiko Fujihara, M.D.
Osaka, Japan
Challenge
- 78-year-old female with severe intermittent claudication (Rutherford category 3)
- Bilateral common iliac artery stenosis (TASC II D lesions) originating at the aortic bifurcation
- Relevant patient history:
- Hypertension, hyperlipidemia, prior bilateral carotid artery stenosis
Procedure
- Gained bilateral access in the common femoral arteries
- Crossed lesions on both the left and right with .014" guidewires
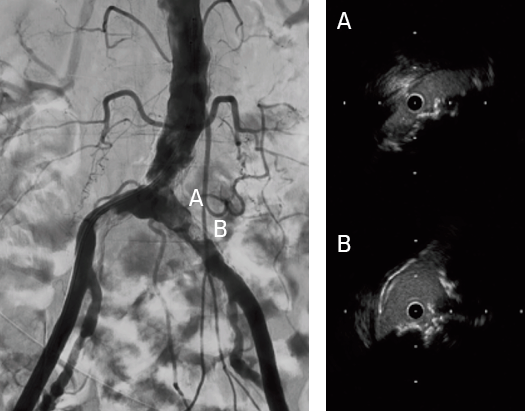
- Dilated with a 2 mm diameter percutaneous transluminal angioplasty (PTA) balloon to allow for intravascular ultrasound examination
- Predilation with 5 mm balloons in kissing balloon technique
- Exchanged to .035" stiff guidewires and 7 Fr long sheaths
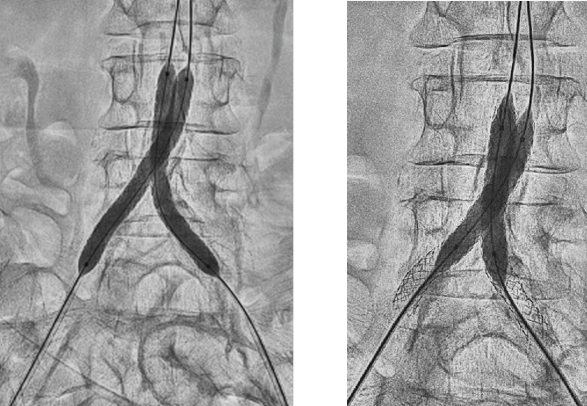
- Deployed two 7 mm x 79 mm GORE® VIABAHN® VBX Balloon Expandable Devices (VBX Stent Graft) using kissing stent technique
- Due to significant calcification, post-dilated first with two 8 mm x 40 mm PTA balloons
- Sequential post-dilation of VBX Stent Grafts proximally with a 10 mm x 20 mm PTA balloon followed by final post-dilation with kissing 8 mm x 40 mm PTA balloons
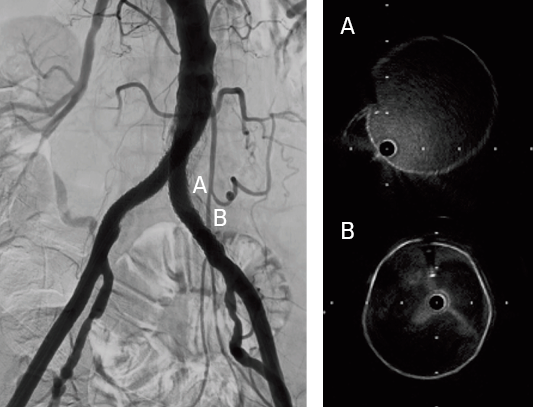
- Confirmed successful result using intravascular ultrasound and angiography
Case Takeaways
The VBX Stent Graft provides durable outcomes in highly calcified lesions. The performance of the VBX Stent Graft allows device customization to the anatomy (6–11 mm for 7 mm x 79 mm device) and provides radial strength to achieve luminal gain while potentially mitigating the risk of rupture and perforation.
- Panneton JM, Bismuth J, Gray BH, Holden A. Three-year follow-up of patients with iliac occlusive disease treated with the Viabahn Balloon-Expandable Endoprosthesis. Journal of Endovascular Therapy 2020;27(5):728-736.
Procedural outcomes based on usage of legacy GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis. (BXA catalogue numbers).
The outcomes and observations reported are based on individual case experience and the patients treated. The steps described here may not be complete, and are not intended to be a replacement for the Instructions for Use or the education, training and professional judgment of health care providers (HCP). HCPs remain solely responsible for making decisions about patient care and the use of medical technologies.

Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. RXOnly
INDICATIONS FOR USE IN THE U.S.: The GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis is indicated for the treatment of de novo or restenotic lesions found in iliac arteries with reference vessel diameters ranging from 5 mm–13 mm and lesion lengths up to 110 mm, including lesions at the aortic bifurcation. The GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis is also indicated for use with thoracoabdominal and pararenal branched devices indicated with the GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis as a branch component.*
CONTRAINDICATIONS: Do not use the GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis in patients with known hypersensitivity to heparin, including those patients who have had a previous incident of Heparin-Induced Thrombocytopenia (HIT) type II.
* Not applicable to Reduced Profile GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis. (BXB catalogue numbers).
The GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis is not authorized for use with thoracoabdominal and pararenal branched devices indicated with the GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis as a branch component in Canada.