Product Value Summary
GORE® VIATORR® TIPS Endoprosthesis with Controlled Expansion

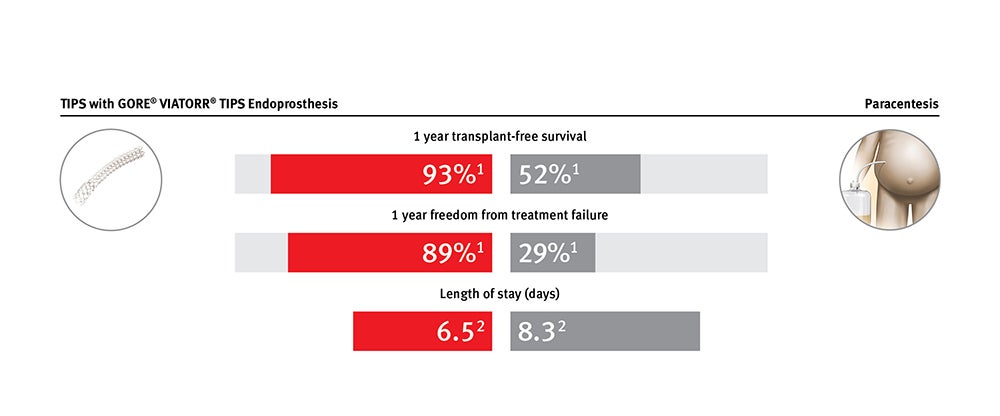
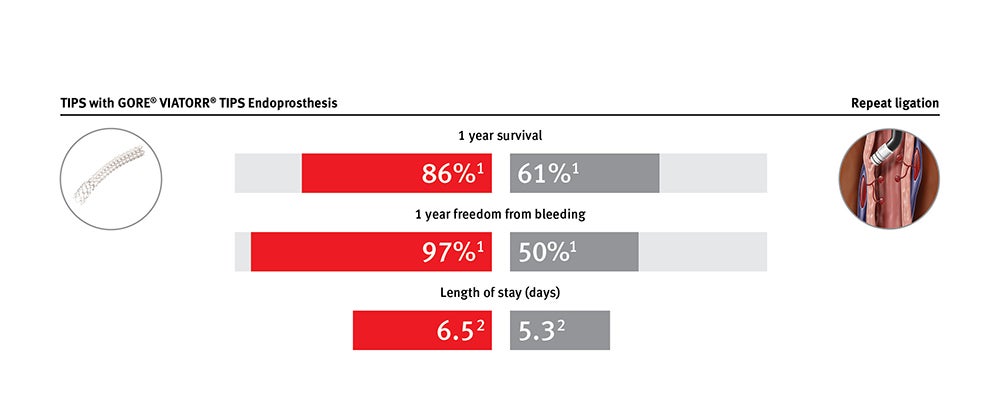
Provides clinical and economic value for treating patients with refractory ascites and variceal bleeding
GORE® VIATORR® Device maintains significantly increased patency compared to bare metal stent alternatives. It is effective in lowering portal pressure gradients in patients with refractory ascites and variceal bleeding.*
The Future of Value Analysis
A Handbook for Health Care Professionals
Read perspectives from value analysis professionals who share their thoughts regarding the importance of effective collaboration, paradigm shifts with determining value, and the critical focus on the future of healthcare.

* Data on file with Gore PMA P040027.
1. Bureau C, Thabut D, Oberti D, et al. Transjugular intrahepatic portosystemic shunts with covered stents increase transplant-free survival of patients with cirrhosis and recurrent ascites. Gastroenterology 2017;152(1):157-163.
http://www.sciencedirect.com/science/article/pii/S0016508516351101.
2. Premier Applied Sciences®, Premier, Inc. Premier Healthcare Database: Data that Informs and Performs. Charlotte, NC: Premier, Inc; 2018. [White paper].
https://learn.premierinc.com/white-papers/premier-healthcare-database-whitepaper.
3. García-Pagán JC, Caca K, Bureau K, et al; Early TIPS (Transjugular Intrahepatic Portosystemic Shunt) Cooperative Study Group. Early use of TIPS in patients with cirrhosis and variceal bleeding. New England Journal of Medicine 2010;362(25):2370-2379. https://www.nejm.org/doi/full/10.1056/NEJMoa0910102.

Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. RXOnly
INTENDED USE/INDICATIONS FOR USE: The GORE® VIATORR® TIPS Endoprosthesis is indicated for use in the de novo and revision treatment of portal hypertension and its complications such as variceal bleeding, gastropathy, refractory ascites and/or hepatic hydrothorax.
CONTRAINDICATIONS: There are no known contraindications for this device.