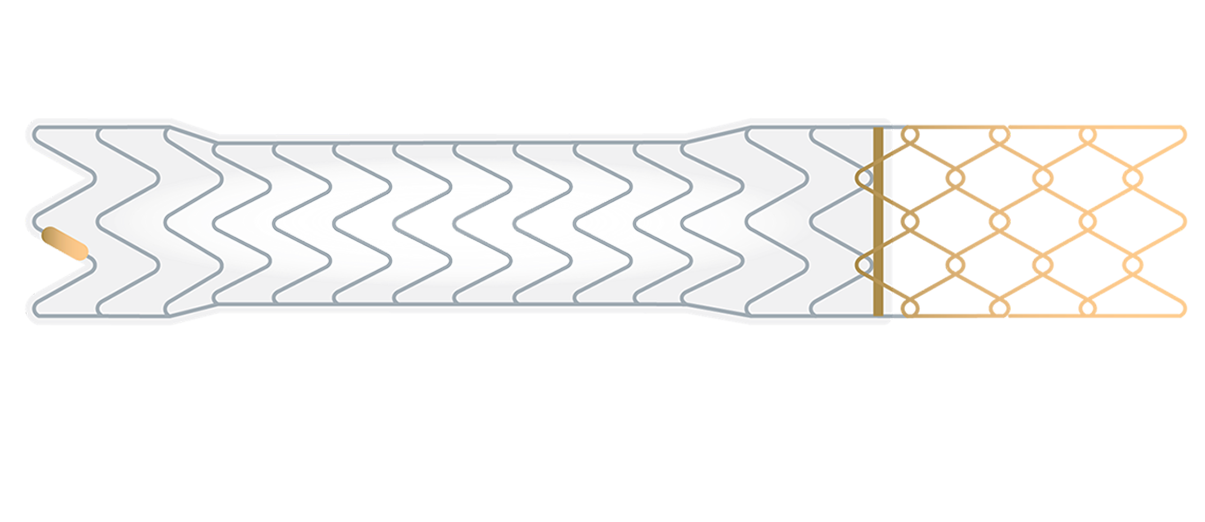
GORE® VIATORR® TIPS Endoprosthesis with Controlled Expansion
Lasting diameter control* and proven patency1

Purpose-built for TIPS
The only covered stent for TIPS with controlled expansion and 25 years of performance and safety data.†
Built for lasting performance
The VIATORR® Device allows you to select and maintain the diameter to reach the targeted portal pressure gradient. Choose a device feature to learn more.
ALTA-recommended for TIPS placement
The American Liver Therapeutic Approaches (ALTA) group recommends "the use of an ePTFE-lined stent graft with controlled expansion, which allows the operator to tailor the amount of portosystemic shunting based on the indication, target gradient and patient comorbidities.”2
Built for long-term results
76%
Two-year primary patency1
The VIATORR® Device was proven to maintain TIPS patency over time in a randomized controlled study (N = 80)1
Built for improved outcomes
Patients treated with the VIATORR® Device have a higher 1-year survival than those treated with non-TIPS therapy.2-4
Variceal bleeding outcomes
Early TIPS compared to drug therapy plus endoscopic band ligation (EBL) for variceal bleeding4
86%
1-year survival
following early TIPS procedure with the VIATORR® Device, compared to 61% 1-year survival following pharmacotherapy plus endoscopic band ligation (P < 0.001)‡,4
97%
1-year control of bleeding
following early TIPS procedure with the VIATORR® Device, compared to 50% 1-year survival following pharmacotherapy plus endoscopic band ligation (P < 0.001)§,4
No increase in the risk of hepatic encephalopathy
at 1 year, compared to pharmacotherapy plus endoscopic band ligation (P = 0.13)4
Refractory ascites outcomes
Early TIPS compared to large volume paracenteses (LVP) and albumin for refractory ascites3
Improved survival
TIPS with the VIATORR® Device demonstrated higher transplant-free survival (93%), compared to large volume paracenteses (LVP) and albumin infusion (52%) (P = .003)3
Fewer paracenteses
with 1 paracentesis procedure required to treat ascites following placement of the VIATORR® Device, compared to 10 procedures with paracentesis treatment alone3
No increase in the risk of hepatic encephalopathy
at 1 year, compared to large volume paracenteses (LVP) and albumin infusion (P = .868)3
Related to this product
* Based on benchtop data on file.
† Historical data from GORE® VIATORR® TIPS Endoprosthesis and GORE® VIATORR® TIPS Endoprosthesis with Controlled Expansion.
‡ For a combined group of patients with Child-Pugh C (CP-C) score ≤13 or Child-Pugh B with active bleeding (CP-B + AB) at diagnostic endoscopy.
§ One-year actuarial probability of remaining free of failure to control bleeding and of variceal rebleeding.
- Bureau C, Pagan JCG, Layrargues GP, et al. Patency of stents covered with polytetrafluoroethylene in patients treated by transjugular intrahepatic portosystemic shunts: long term results of a randomized multicentre study. Liver International 2007;27(6):742-747.
- Boike JR, Thornburg BG, Asrani SK, et al. North American Practice-Based Recommendations for Transjugular Intrahepatic Portosystemic Shunts in Portal Hypertension. Clinical Gastroenterology and Hepatology. 2022;20(8):1636-1662.e36.
- Bureau C, Thabut D, Oberti F, et al. Transjugular intrahepatic portosystemic shunts with covered stents increase transplant-free survival of patients with cirrhosis and recurrent ascites. Gastroenterology 2017;152(1):157-163.
- García-Pagán JC, Caca K, Bureau C, et al. Early use of TIPS in patients with cirrhosis and variceal bleeding. The New England Journal of Medicine 2010;362(25):2370-2379.

Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. RXOnly
INDICATIONS FOR USE IN THE U.S.: The GORE® VIATORR® TIPS Endoprosthesis is indicated for use in the de novo and revision treatment of portal hypertension and its complications such as variceal bleeding, gastropathy, ascites which recurs despite conventional treatment, and/or hepatic hydrothorax.
INDICATIONS FOR USE IN CANADA: The GORE® VIATORR® TIPS Endoprosthesis with Controlled Expansion is indicated for the treatment of portal hypertension and its complications such as variceal bleeding and refractory ascites.
CONTRAINDICATIONS: There are no known contraindications for this device.