Accessories for GORE® TAG® Conformable Thoracic Stent Graft with ACTIVE CONTROL System
The next generation in aortic accessories
Each product in the aortic accessory portfolio plays a key role in supporting our best-in-class EVAR and TEVAR devices in promoting positive outcomes.
GORE® DRYSEAL Flex Introducer Sheath
- Deliver with ease: Hydrophilic coating and enhanced flexibility provide exceptional access to challenging anatomies and branch vessels.
- Minimize blood loss: Exclusive GORE® DRYSEAL Valve enables introduction of multiple devices with proven hemostasis control.
- Care for more patients: Optimized profile and configurations provide tailored delivery options for a broad range of patient anatomy.
- Complete confidence: Engineered for use with our endovascular portfolio.

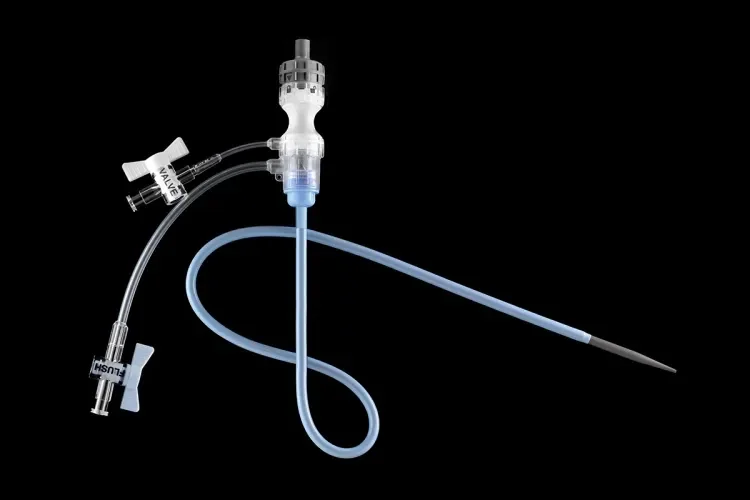
GORE® Tri-Lobe Balloon Catheter
- Continuous blood flow: Unique design allows flow around the lobes while inflated.
- Decreased hemodynamic pressure on the inflated balloons with approximately 80% of flow for distal perfusion.1
- Minimizes potential for endoprosthesis movement and blood pressure spikes post deployment due to significantly reduced hemodynamic pressures.2
- Rapid, uniform and simultaneous inflation and deflation.
- Bloss R, Krall B. Balloon Testing on Pulse Duplicator. Flagstaff, AZ: W. L. Gore & Associates, Inc.; 2009. [Technology notebook]. 1088.
- Gendron M. Simulated Use Testing of Aortic Balloon Catheter for Design Verification – Final Amendment. Flagstaff, AZ: W. L. Gore & Associates, Inc; 2008. [Work plan]. MD39672.
INDICATIONS FOR USE IN THE U.S.: The GORE® DRYSEAL Flex Introducer Sheath is intended to be inserted in the vasculature to provide a conduit for the insertion of endovascular devices while minimizing blood loss associated with such insertions. CONTRAINDICATIONS: There are no known contraindications for this device. Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. RXOnly
INDICATIONS FOR USE IN THE U.S.: The GORE® Tri-Lobe Balloon Catheter is indicated to facilitate in the endovascular repair of the thoracic or abdominal aorta due to lesions including aneurysms, dissections, trauma, and penetrating aortic ulcers. CONTRAINDICATIONS: There are no known contraindications. Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. RXOnly
Sizing, availability, and pricing varies by country. Please check with your Gore representative for availability.
24AR1072-EN01