The device behind the data
GORE® TAG® Conformable Thoracic Stent Graft with ACTIVE CONTROL System
Designed for the demands of TEVAR
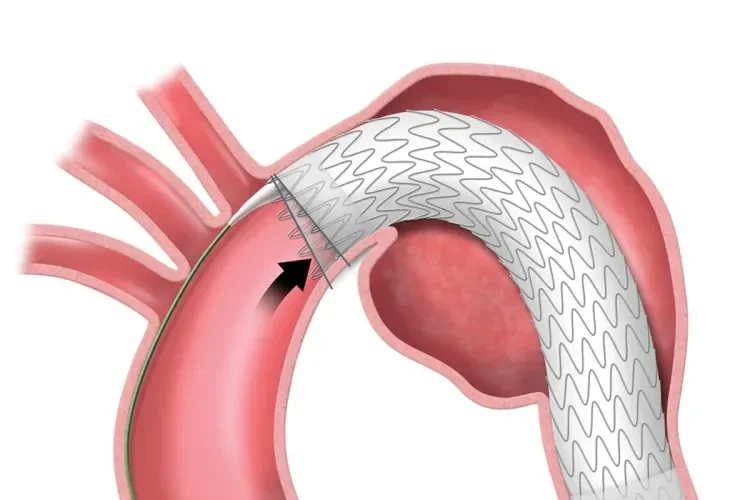
The TAG® Conformable Device combines a trusted stent graft with a unique delivery system designed for the complex, angulated anatomy of the thoracic aorta.
The difference is precision
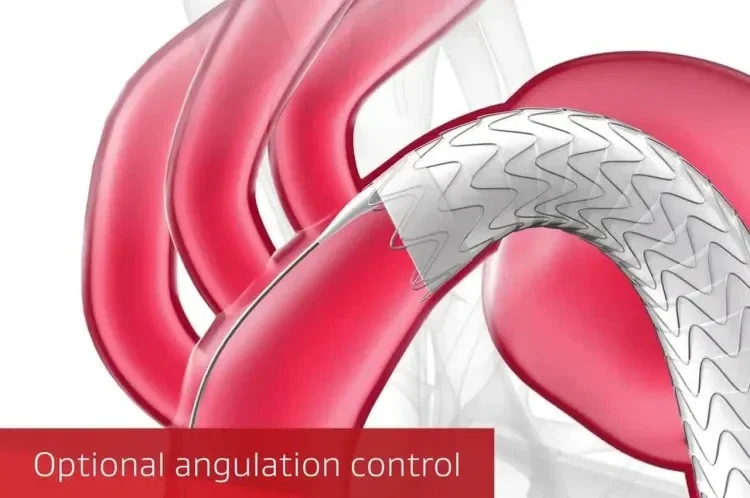
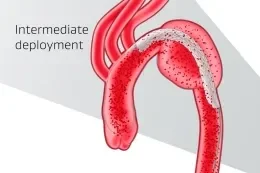
Unique angulation control is available at both intermediate and full deployment, empowering you with the precision to adjust for 360° wall apposition and seal along inner curve of the aorta.

98% reported that proximal wall apposition was acceptable at procedural completion1
The difference is conformability
Enhanced control and exceptional conformability combine to help minimize the risk of adverse events requiring reintervention.

Zero Type III endoleaks reported in 5-year data across all etiologies*,2-4
Setting a standard of conformability
The TAG® Conformable Device exhibited the lowest spring back in bench-top analysis.
Benchtop evaluations are intended to demonstrate relative physical characteristics and may not correlate to clinical results. All devices were tested under equivalent situations. Complete information and data are on file.
Adapting to aortic anatomy
Our device adapts to the aorta by design, rather than the aorta adapting to the device. See the impact in real patient anatomy over 5 years of follow-up:

Pre-treatment

1 Year

2 Years
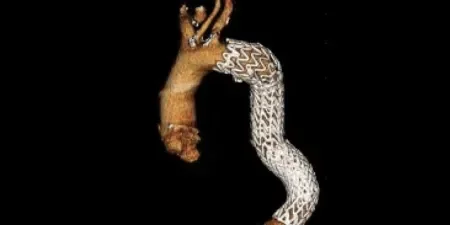
3 Years
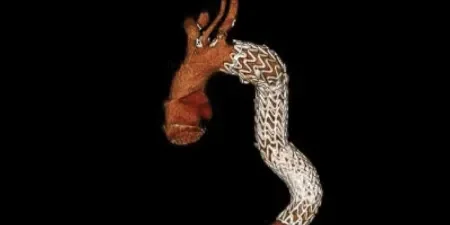
4 Years

5 Years
The difference is meaningful
The TAG® Conformable Device harnesses more than 25 years of TEVAR experience and evidence.
That’s just one number worth counting on:

Zero migrations,† fractures or compressions* reported in 5 years across all etiologies

1st device approved for endovascular treatment of all etiologies

1st TEVAR solution to feature angulation control

10+ years of follow-up
* No reported compressions, fractures or Type III endoleaks.
† Migrations are reported as those requiring reintervention.
- W. L. Gore & Associates. Observational Registry Characterizing the Performance and Feature Use of the GORE® TAG® Conformable Thoracic Stent Graft Featuring ACTIVE CONTROL System. NLM Identifier: NCT03286400. Published September 18, 2017. Updated December 17, 2020. Accessed February 23, 2021. https://clinicaltrials.gov/ct2/show/NCT03286400
- W. L. Gore & Associates. Evaluation of the GORE Conformable TAG® Thoracic Endoprosthesis for Treatment of Acute Complicated Type B Aortic Dissection. NLM Identifier: NCT00908388. Published May 25, 2009. Updated October 27, 2017. Accessed June 7, 2022. https://clinicaltrials.gov/ct2/show/NCT00908388
- W. L. Gore & Associates. Evaluation of the GORE Conformable TAG® for Treatment of Traumatic Transection. NLM Identifier: NCT00917852. Published June 10, 2009. Updated August 25, 2017. Accessed June 7, 2022. https://clinicaltrials.gov/ct2/show/NCT00917852
- W. L. Gore & Associates. An Evaluation of the GORE Conformable TAG® Thoracic Endoprosthesis for the Primary Treatment of Aneurysm of the Descending Thoracic Aortic. NLM Identifier: NCT00874250. Published April 2, 2009. Updated August 25, 2017. Accessed June 7, 2022. https://clinicaltrials.gov/ct2/show/NCT00874250
Related to this product

INDICATIONS FOR USE IN THE U.S.: The GORE® TAG® Conformable Thoracic Stent Graft is intended for endovascular repair of all lesions of the descending thoracic aorta, including: isolated lesions in patients who have appropriate anatomy, including: adequate iliac/femoral access, aortic inner diameter in the range of 16-42 mm, ≥ 20 mm non-aneurysmal aorta proximal and distal to the lesion; Type B dissections in patients who have appropriate anatomy, including: adequate iliac/femoral access, ≥ 20 mm landing zone proximal to the primary entry tear; proximal extent of the landing zone must not be dissected, diameter at proximal extent of proximal landing zone in the range of 16-42 mm. CONTRAINDICATIONS: Patients with known sensitivities or allergies to the device materials; patients who have a condition that threatens to infect the graft. Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. RXOnly
24AR1091-EN01