The device behind the data
GORE® TAG® Conformable Thoracic Stent Graft with ACTIVE CONTROL System
Designed for the demands of TEVAR
The TAG® Conformable Device combines a trusted stent graft with a unique delivery system designed for the complex, angulated anatomy of the thoracic aorta.
The difference is control
The GORE® ACTIVE CONTROL System provides an intermediate stage before full deployment—at 50% diameter—giving you the confidence of control.
Visualize, correct for parallax, reposition and refine angulation, all in real time.
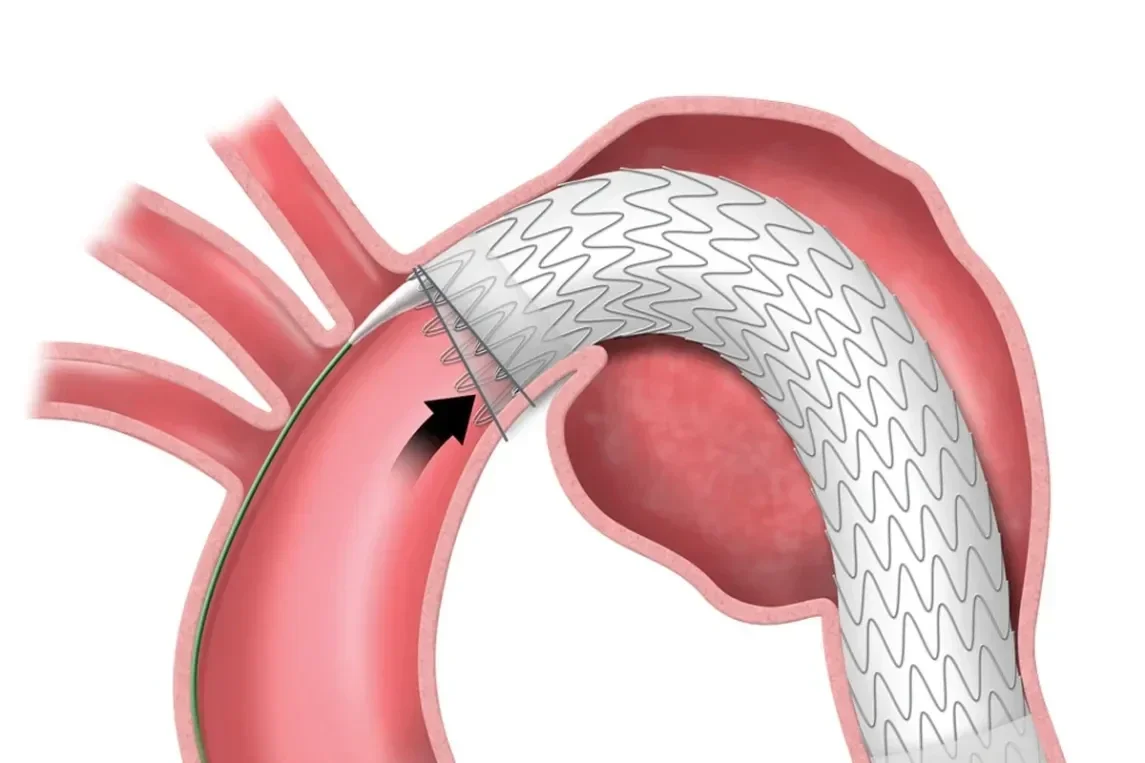
Angulation adjustment at full deployment.
The difference is precision
Unique angulation control is available at both intermediate and full deployment, empowering you with the precision to adjust for 360° wall apposition and seal along inner curve of the aorta.
98%
reported that proximal wall apposition was acceptable at procedural completion1
Deliver and deploy with precision in complex anatomy.
The difference is conformability
Enhanced control and exceptional conformability combine to help minimize the risk of adverse events requiring reintervention.
Zero Type III endoleaks
reported in 5-year data across all etiologies*,2-4
Setting a standard of conformability
The TAG® Conformable Device exhibited the lowest spring back in bench-top analysis.
Benchtop evaluations are intended to demonstrate relative physical characteristics and may not correlate to clinical results. All devices were tested under equivalent situations. Complete information and data are on file.
The difference is meaningful
The TAG® Conformable Device harnesses more than 25 years of TEVAR experience and evidence.
That’s just one number worth counting on:

ZERO
migrations,† fractures or compressions* reported in 5 years across all etiologies
1st device
approved for endovascular treatment of all etiologies
1st TEVAR solution
to feature angulation control
10+
years of follow-up
Related to this product
* No reported compressions, fractures or Type III endoleaks.
† Migrations are reported as those requiring reintervention.
W. L. Gore & Associates. Observational Registry Characterizing the Performance and Feature Use of the GORE® TAG® Conformable Thoracic Stent Graft Featuring ACTIVE CONTROL System. NLM Identifier: NCT03286400. Published September 18, 2017. Updated December 17, 2020. Accessed February 23, 2021. https://clinicaltrials.gov/ct2/show/NCT03286400
W. L. Gore & Associates. Evaluation of the GORE Conformable TAG® Thoracic Endoprosthesis for Treatment of Acute Complicated Type B Aortic Dissection. NLM Identifier: NCT00908388. Published May 25, 2009. Updated October 27, 2017. Accessed June 7, 2022. https://clinicaltrials.gov/ct2/show/NCT00908388
W. L. Gore & Associates. Evaluation of the GORE Conformable TAG® for Treatment of Traumatic Transection. NLM Identifier: NCT00917852. Published June 10, 2009. Updated August 25, 2017. Accessed June 7, 2022. https://clinicaltrials.gov/ct2/show/NCT00917852
W. L. Gore & Associates. An Evaluation of the GORE Conformable TAG® Thoracic Endoprosthesis for the Primary Treatment of Aneurysm of the Descending Thoracic Aortic. NLM Identifier: NCT00874250. Published April 2, 2009. Updated August 25, 2017. Accessed June 7, 2022. https://clinicaltrials.gov/ct2/show/NCT00874250

Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. RXOnly
INDICATIONS FOR USE IN THE U.S.: The GORE® TAG® Conformable Thoracic Stent Graft is intended for endovascular repair of all lesions of the descending thoracic aorta, including: isolated lesions in patients who have appropriate anatomy, including: adequate iliac/femoral access, aortic inner diameter in the range of 16-42 mm, ≥ 20 mm non-aneurysmal aorta proximal and distal to the lesion; Type B dissections in patients who have appropriate anatomy, including: adequate iliac/femoral access, ≥ 20 mm landing zone proximal to the primary entry tear; proximal extent of the landing zone must not be dissected, diameter at proximal extent of proximal landing zone in the range of 16-42 mm.
CONTRAINDICATIONS: Patients with known sensitivities or allergies to the device materials; patients who have a condition that threatens to infect the graft.







