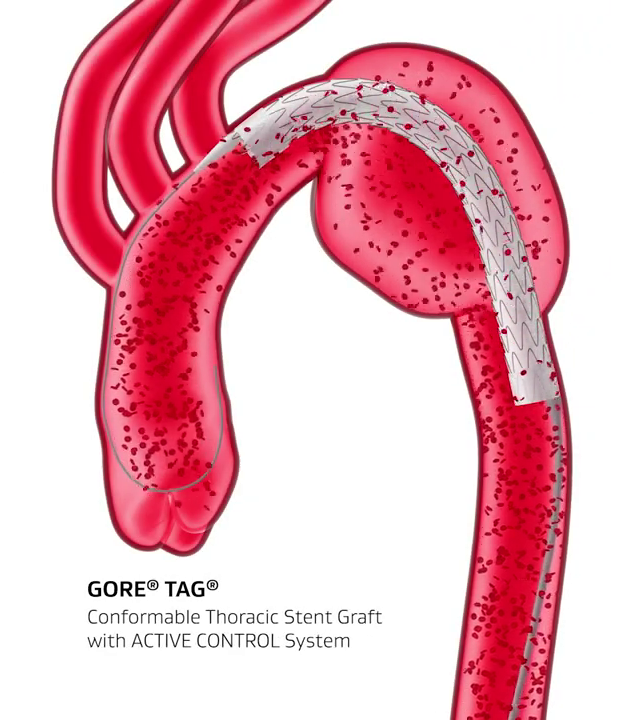
Hemodynamic Stability with the GORE® TAG® Conformable Thoracic Stent Graft with ACTIVE CONTROL System
At intermediate stage you can adjust positioning and angulation, while allowing continuous blood flow throughout the procedure.
The TAG® Conformable Device combines a durable, proven stent graft with a delivery system that offers controlled, staged deployment.
At the intermediate deployment phase, the stent graft is not at full diameter, allowing for continuous blood flow through the aorta. This promotes hemodynamic stability throughout the procedure, which minimizes “windsocking.” It also reduces the need for aggressive blood pressure management or use of rapid pacing.
Hemodynamic Flow Animation
Demonstrates the continuous blood flow throughout deployment of the stent graft
Related to this product

Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. RXOnly
INDICATIONS FOR USE IN THE U.S.: The GORE® TAG® Conformable Thoracic Stent Graft is intended for endovascular repair of all lesions of the descending thoracic aorta, including: isolated lesions in patients who have appropriate anatomy, including: adequate iliac/femoral access, aortic inner diameter in the range of 16-42 mm, ≥ 20 mm non-aneurysmal aorta proximal and distal to the lesion; Type B dissections in patients who have appropriate anatomy, including: adequate iliac/femoral access, ≥ 20 mm landing zone proximal to the primary entry tear; proximal extent of the landing zone must not be dissected, diameter at proximal extent of proximal landing zone in the range of 16-42 mm.
CONTRAINDICATIONS: Patients with known sensitivities or allergies to the device materials; patients who have a condition that threatens to infect the graft.