In-stent restenosis of the SFA
Consider the complexity and therapeutic challenge of in-stent restenosis (ISR) in the superficial femoral artery (SFA)
- ISR is the “Achilles' heel” of peripheral vascular interventions1
- Reported to occur within a year in up to 45% of femoropopliteal lesions treated with bare metal stents2-4
- Lesion length is a risk factor for ISR for bare metal and drug-eluting stents5-6
- There are a limited number of multicenter, prospective randomized ISR trials7-10
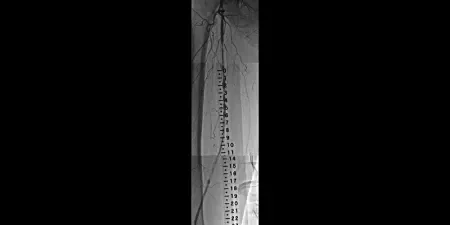
Before
Diffuse SFA in-stent restenosis in long- stented segment in the SFA
Images courtesy of Robert Minor, M.D. Used with permission.
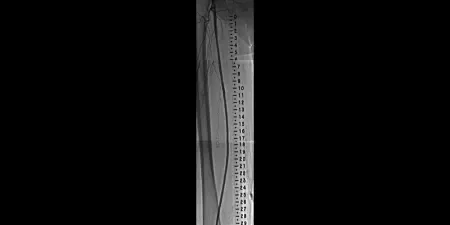
After
Completion angiogram after placement of GORE® VIABAHN® Devices for in-stent restenosis in the SFA
Images courtesy of Robert Minor, M.D. Used with permission.
The GORE® VIABAHN® Endoprosthesis with PROPATEN Bioactive Surface*,† delivers exceptional patency for in-stent restenosis lesions.10
Results from the Gore RELINE Clinical Study, a multicenter, prospective, randomized, controlled trial10
Proven patency
- 75% one-year primary patency compared to 28% percutaneous transluminal angioplasty (PTA)10
- Fewer than one third the number of patients required an intervention at one year compared to PTA.11
Demonstrated durability
- Four times greater primary patency compared to PTA at two years 12
- More than three times greater freedom from target lesion revascularization (fTLR) compared to PTA at two years11
Fewer Reinterventions
- Fewer patients had reintervention procedures compared to PTA at two years11
Efficacy in complex lesions and in a difficult patient population
- The average lesion length in the Gore RELINE Clinical Study exceeded 17 cm10
- In the same study, approximately 21% of patients had occlusions10
…[In] my clinical experience, the GORE® VIABAHN® [Endoprosthesis] is an excellent choice for treating long-segment de novo as well as in-stent restenotic occlusions13
-Robert Bersin, M.D., Seattle, Washington.
Connect with a Gore Field Sales Associate
* As used by Gore, PROPATEN Bioactive Surface refers to Gore's proprietary CBAS Heparin Surface.
† Also referred to as the GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface in some regions.
- Adams GL, Bersin RM, George JC, Subramanian V, Soukas PA. Data-driven treatment approach to in-stent restenosis. Endovascular Today 2015;14(6)Supplement:3-8.
- Bosiers M, Torsello G, Gissler HM, et al. Nitinol stent implantation in long superficial femoral artery lesions: 12-month results of the DURABILITY I study. Journal of Endovascular Therapy 2009;16(3):261-269.
- Krankenberg H, Schlüter M, Steinkamp HJ, et al. Nitinol stent implantation versus percutaneous transluminal angioplasty in superficial femoral artery lesions up to 10 cm in length: the femoral artery stenting trial (FAST). Circulation 2007;116(3):285-292.
- Lammer J, Zeller T, Hausegger KA, et al. Heparin-bonded covered stents versus bare-metal stents for complex femoropopliteal artery lesions: the randomized VIASTAR trial (Viabahn endoprosthesis with PROPATEN bioactive surface [VIA] versus bare nitinol stent in the treatment of long lesions in superficial femoral artery occlusive disease). Journal of the American College of Cardiology 2013;62(15):1320-1327.
- Gao M, Zhao X, Tao Y, et al. Incidence and predictors of in-stent re-stenosis in the superficial femoral artery: evaluation of long-term outcomes by color duplex ultrasound. Ultrasound in Medicine & Biology 2016;42(3):717-726.
- Iida O, Takahara M, Soga Y, et al; ZEPHYR Investigators. 1-year results of the ZEPHYR Registry (Zilver PTX for the Femoral Artery and Proximal Popliteal Artery): predictors of restenosis. JACC: Cardiovascular Interventions 2015;8(8):1105-1112.
- Krankenberg H; FAIR Trial Investigators. DCB and ISR: insights from the FAIR Study. Presented at the Leipzig Interventional Course (LINC); January 27-29, 2015; Leipzig, Germany.
- Lutonix® 035 Drug Coated Balloon PTA Catheter [Instructions for Use]. New Hope, MN: Lutonix, Inc; 2016. BAW1387400 Rev. 1.
- Dippel EJ; EXCITE ISR Investigators. EXCITE ISR: initial results. Presented at the Transcatheter Cardiovascular Therapeutics Twenty‑Sixth Annual Symposium Transcatheter Cardiovascular Therapeutics (TCT); September 13‑17, 2014; Washington, DC.
- Bosiers M, Deloose K, Callaert J, et al. Superiority of stent‑grafts for in‑stent restenosis in the superficial femoral artery: twelve‑month results from a multicenter randomized trial. Journal of Endovascular Therapy 2015;22(1):1-10.
- GORE® VIABAHN® Endoprosthesis with PROPATEN Bioactive Surface [Instructions for Use]. Flagstaff, AZ: W. L. Gore & Associates, Inc; 2021. MD174703.
- Bosiers M, Deloose K. 2-year results of the RELINE Trial comparing Viabahn Stent-Grafts to POBA for ISR show durable benefits to endograft treatment. Presented at the 41st Annual Symposium on Vascular and Endovascular Issues, Techniques, Horizons (VEITHsymposium); November 18-22, 2014; New York, NY. Abstract 168.
- Bersin RM. Treating ISR with the GORE® VIABAHN® Endoprosthesis after bilateral SFA CTO interventions. Endovascular Today 2015;14(6)Supplement:13-14. https://evtoday.com/pdfs/et0615_GORE_supplement_viabahn_sec%204.pdf