Chronic total SFA occlusions
Consider the complexity of treating chronic total occlusions (CTOs)
- CTOs can increase the need for provisional stenting1
- In the Medtronic IN.PACT Global Study, treatment of CTOs increased the rate of provisional stenting by 85%
- Although a rare complication, the risk of distal embolization has been shown to increase when treating a CTO2-3
- Limited comparison data exists between newer endovascular technologies compared to surgical bypass, the current recommended treatment for CTOs4
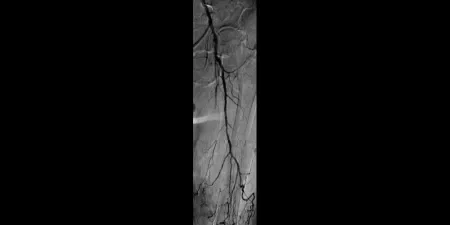
Before
Left SFA CTO with one-vessel runoff (not shown)
Images courtesy of Bruce Gray, M.D. Used with Permission.
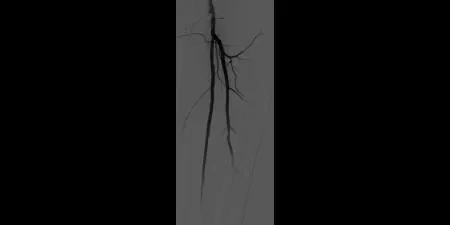
After
Post-placement of two 6 mm x 15 cm GORE® VIABAHN® Devices. Maintained one-vessel runoff. (not shown)
Images courtesy of Bruce Gray, M.D. Used with Permission.
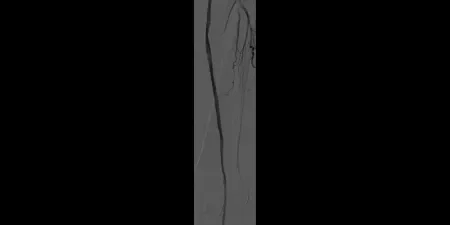
After
Post-placement of two 6 mm x 15 cm GORE® VIABAHN® Devices. Maintained one-vessel runoff. (not shown)
Images courtesy of Bruce Gray, M.D. Used with Permission.
The GORE® VIABAHN® Endoprosthesis with PROPATEN Bioactive Surface*,† has been extensively studied in CTOs5-11
Proven patency
- 80% average primary patency was achieved across seven multicenter, prospective, randomized or single arm studies, of which 71% of the limbs studied were CTOs (774/1089).5-11
Demonstrated durability
- 97% three-year secondary patency in complex disease (27 cm average lesion length, 93% CTOs)12
- Comparable clinical results to above knee surgical bypass (both prosthetic5 and native vein13)
The GORE® VIABAHN® Endoprosthesis design is uniquely capable of treating CTOs
- Expanded PTFE (ePTFE) flow lumen covers and seals off residual thrombosis
- CBAS Heparin Surface, also featured in the GORE® PROPATEN® Vascular Graft, is the proven, lasting heparin bonding technology designed to resist thrombus formation14
Connect with a Gore Field Sales Associate
* As used by Gore, PROPATEN Bioactive Surface refers to Gore's proprietary CBAS Heparin Surface.
† Also referred to as the GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface in some regions.
- Brodmann M. Real world value of the In.Pact Admiral DCB (Medtronic) for fem-pop lesions: from the In.Pact Global Registry: what else does it tell us. Presented at the 44th Annual Symposium on Vascular and Endovascular Issues, Techniques, Horizons (VEITH symposium); November 14-18, 2017; New York, NY.
- Wu W, Hua S, Li Y, et al. Incidence, risk factors, treatment and prognosis of popliteal artery embolization in the superficial femoral artery interventions. PLoS One 2014;9(9):e107717.
- Shrikhande GV, Khan SZ, Hussain HG, Dayal R, McKinsey JF, Morrissey N. Lesion types and device characteristics that predict distal embolization during percutaneous lower extremity interventions. Journal of Vascular Surgery 2011;53(2):347-352.
- Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG; TASC II Working Group. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). Journal of Vascular Surgery 2007;45(1)Supplement:S5-S67.
- Reijnen M, van Walraven L, Fritschy W, et al. 1-year results of a multicenter, randomized controlled trial comparing heparin-bonded endoluminal to femoropopliteal bypass. Journal of Cardiovascular Interventions 2017;10(22):2320-2331.
- Ohki T, Kichikawa K, Yokoi H, et al. Long-term results of the Japanese multicenter Viabahn trial of heparin bonded endovascular stent grafts for long and complex lesions in the superficial femoral artery. Journal of Vascular Surgery. In press.
- Saxon RR, Chervu A, Jones PA, et al. Heparin‑bonded, expanded polytetrafluoroethylene‑lined stent graft in the treatment of femoropopliteal artery disease: 1‑year results of the VIPER (Viabahn Endoprosthesis with Heparin Bioactive Surface in the Treatment of Superficial Femoral Artery Obstructive Disease) Trial. Journal of Vascular & Interventional Radiology 2013;24(2):165-173.
- Lammer J, Zeller T, Hausegger KA, et al. Sustained benefit at 2 years for covered stents versus bare-metal stents in long SFA lesions: the VIASTAR trial. Cardiovascular & Interventional Radiology 2015;38(1):25-32.
- Zeller T, Peeters P, Bosiers M, et al. Heparin‑bonded stent‑graft for the treatment of TASC II C and D femoropopliteal lesions: the Viabahn‑25 cm Trial. Journal of Endovascular Therapy 2014;21(6):765‑774.
- Iida O. Twelve-month outcomes from the Japanese post-market surveillance study of the GORE® VIABAHN® Endoprosthesis as treatment for symptomatic peripheral arterial disease in the superficial femoral arteries. Presented at LEIPZIG Interventional Course (LINC)2021; January 25-29, 2021; Leipzig, Germany.
- Iida O, Takahara M, Soga Y, et al; VANQUISH Investigators. One-year outcomes of heparin-bonded stent-graft therapy for real-world femoropopliteal lesions and the association of patency with the prothrombotic state based on the prospective, observational, multicenter Viabahn Stent-Graft Placement for Femoropopliteal Diseases Requiring Endovascular Therapy (VANQUISH) Study. Journal of Endovascular Therapy 2021;28(1):123-131.
- GORE® VIABAHN® Endoprosthesis with PROPATEN Bioactive Surface [Instructions for Use]. Flagstaff, AZ: W. L. Gore & Associates, Inc; 2021. MD174703.
- McQuade K, Gable D, Pearl G, Theune B, Black S. Four-year randomized prospective comparison of percutaneous ePTFE/nitinol self-expanding stent graft versus prosthetic femoral-popliteal bypass in the treatment of superficial femoral artery occlusive disease. Journal of Vascular Surgery 2010;52(3):584-591.
- CBAS Heparin Surface. W. L. Gore & Associates website. Accessed October 28, 2020. https://www.goremedical.com/cbas/references