Devices
Join the next generation of care in the treatment of aortoiliac occlusive disease
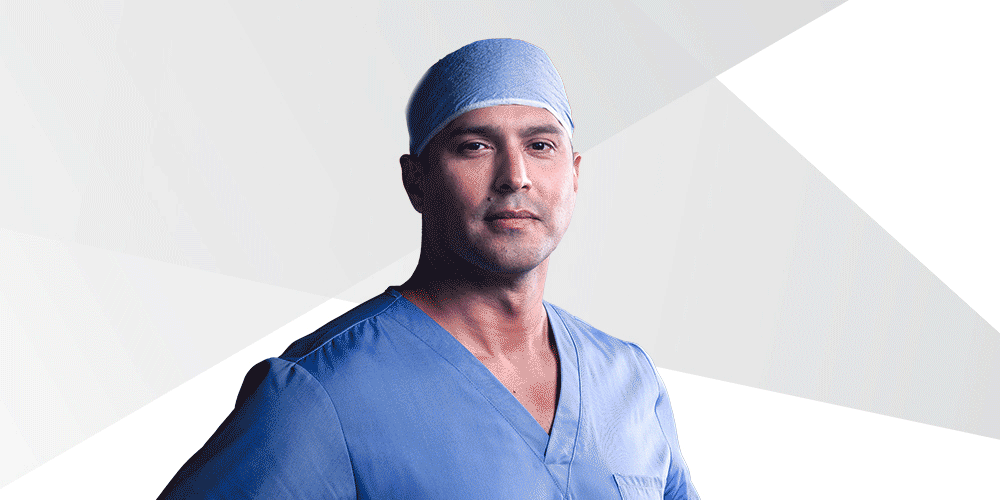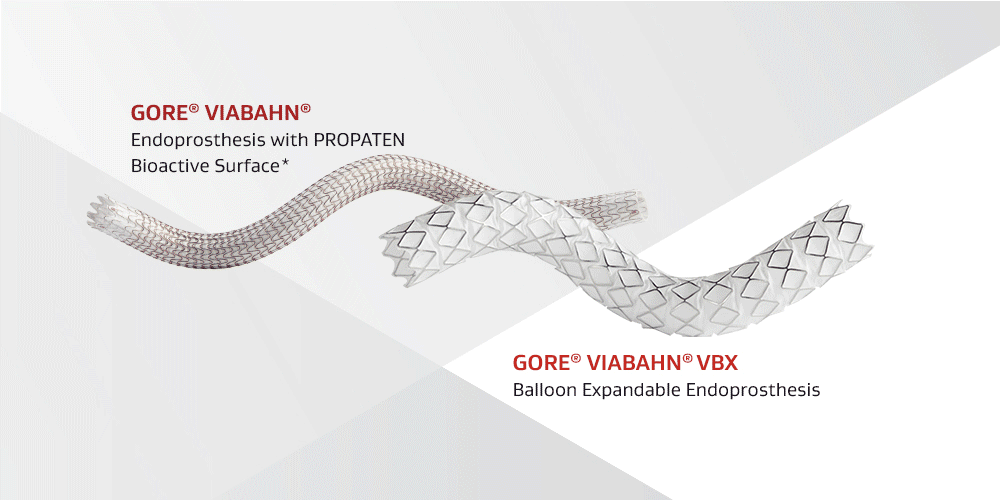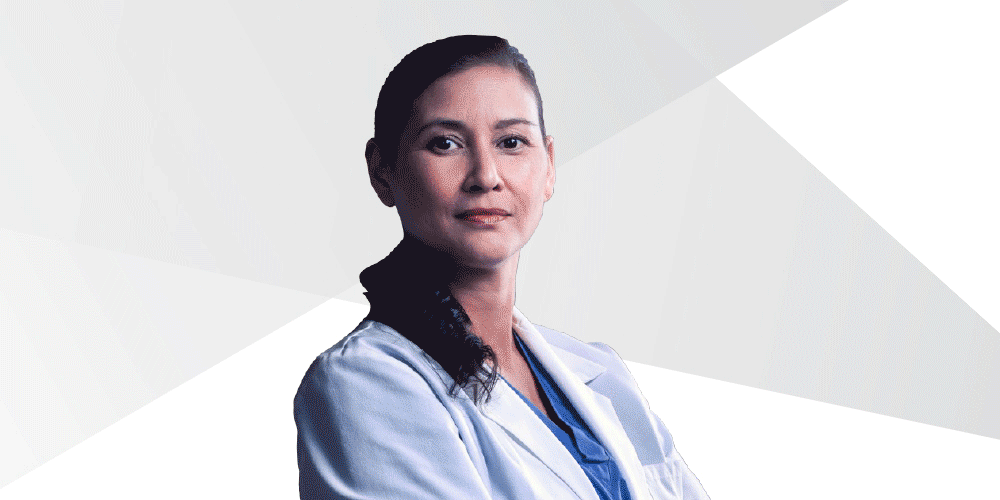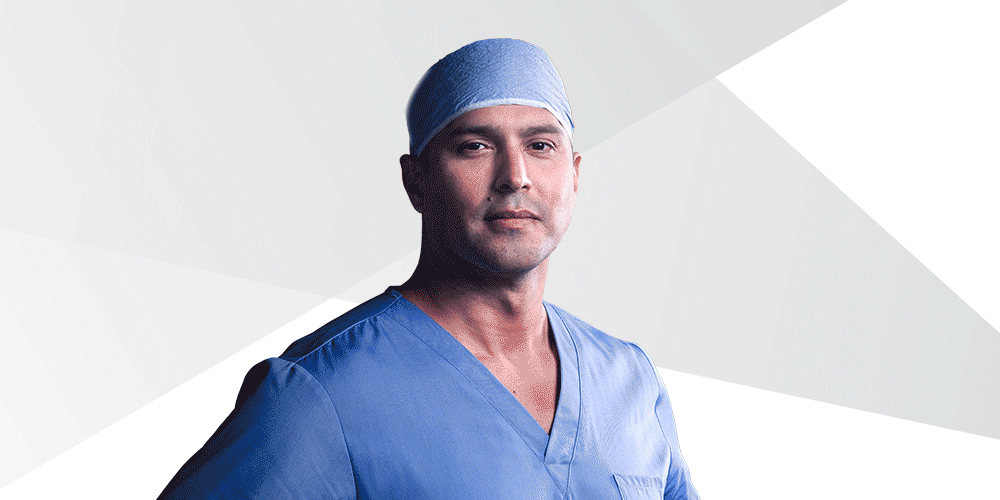
The latest generation of covered stent grafts
GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis (VBX Stent Graft)
GORE® VIABAHN® Endoprosthesis with PROPATEN Bioactive Surface* (GORE® VIABAHN® Device)
VBX Stent Graft
Decide with data
5-year follow-up shows the VBX Stent Graft continues to be a long-term durable treatment option for complex aortoiliac occlusive disease (AIOD).1
The Gore VBX FLEX Clinical Study 3-year follow-up found the VBX Stent Graft to be a robust and durable treatment option for AIOD.2 Now, sustained patient benefit and durability are demonstrated through 5 years.
Physician-initiated 5-year VBX Stent Graft follow-up
Objective and methodology1
- This physician-initiated study enrolled 59 of the initial 134 patients from 3 participating centers that were representative of the VBX FLEX Study 3-year follow-up cohort. 28 patients completed the 5-year follow-up.
- The primary durability endpoint was long-term primary patency.
DURABLE CLINICAL OUTCOMES THROUGH 5 YEARS1:
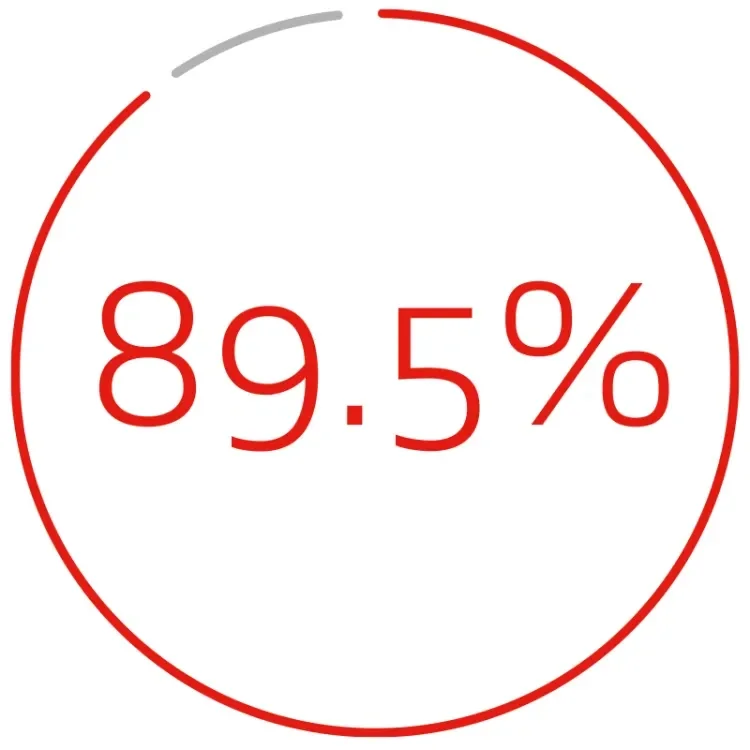
primary patency per lesion
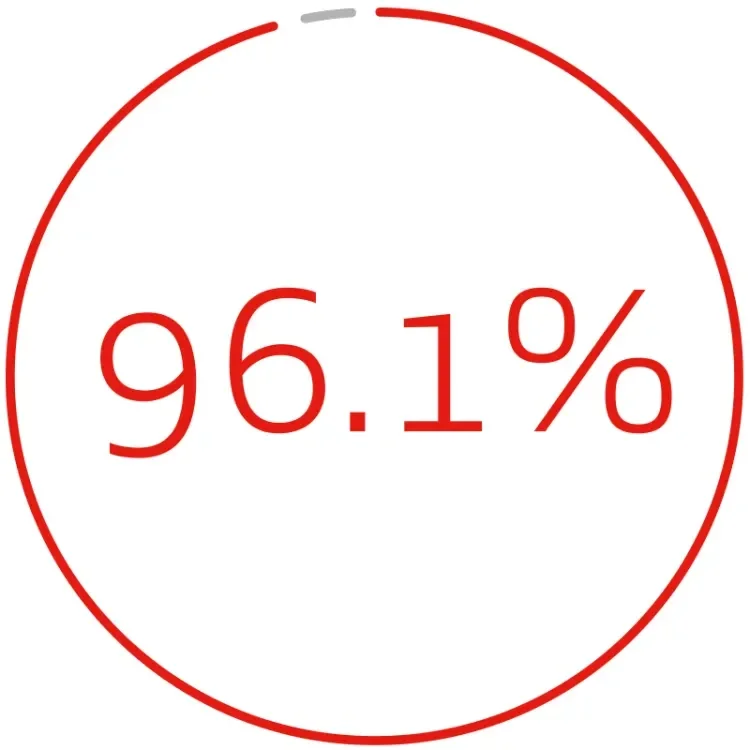
primary assisted patency per lesion
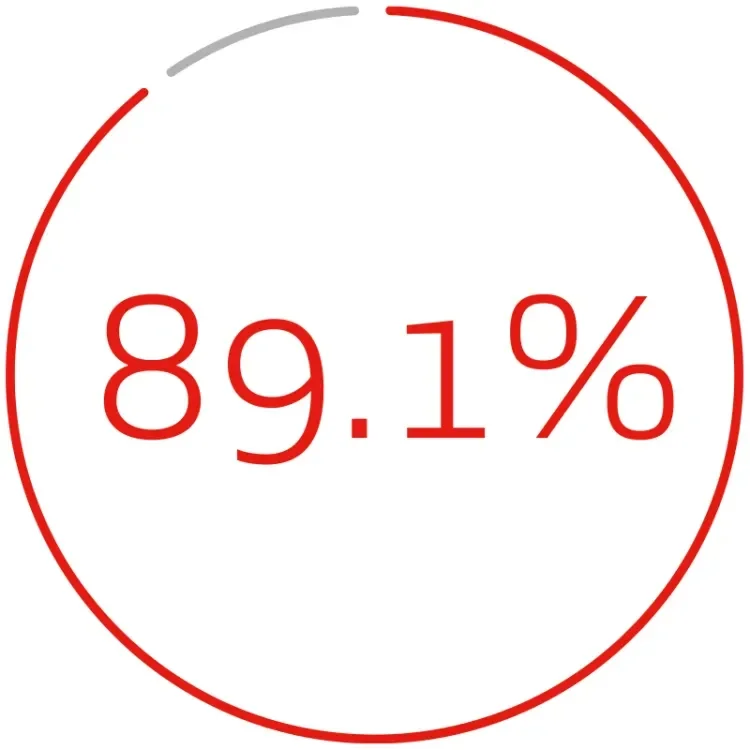
freedom from target lesion revascularization per subject
SUSTAINED PATIENT BENEFIT THROUGH 5 YEARS1:

of evaluated (n=28) patients improved ≥ 1 Rutherford category from baseline†,1
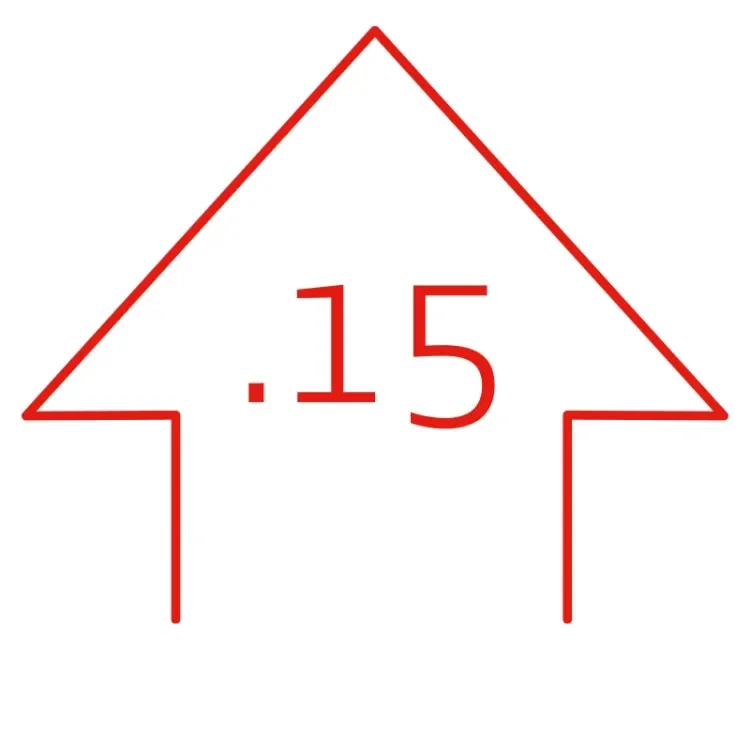
improvement in mean resting ankle-brachial index (ABI)
(P < .001, .95 mean ABI) ‡
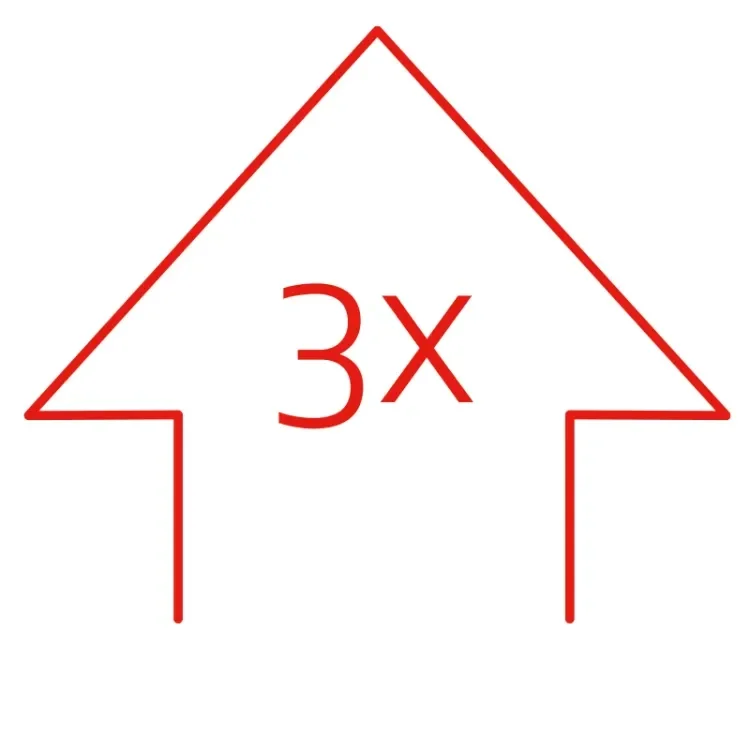
improvement in median WIQ measures
The case for long-term durable clinical outcomes:
Case study 1: Restoring flow to a patient with stenosis at the aortoiliac bifurcation
Case study 2: Restoring flow in a patient with severe claudication and focal iliac calcification
GORE® VIABAHN® Device
Durable clinical outcomes and proven patency even in complex cases.§

GORE® VIABAHN® Device outcomes in iliac occlusive disease
Retrospective multicenter study, evaluating the performance of the GORE® VIABAHN® Device (n = 93) for the treatment of external iliac artery obstructive disease.
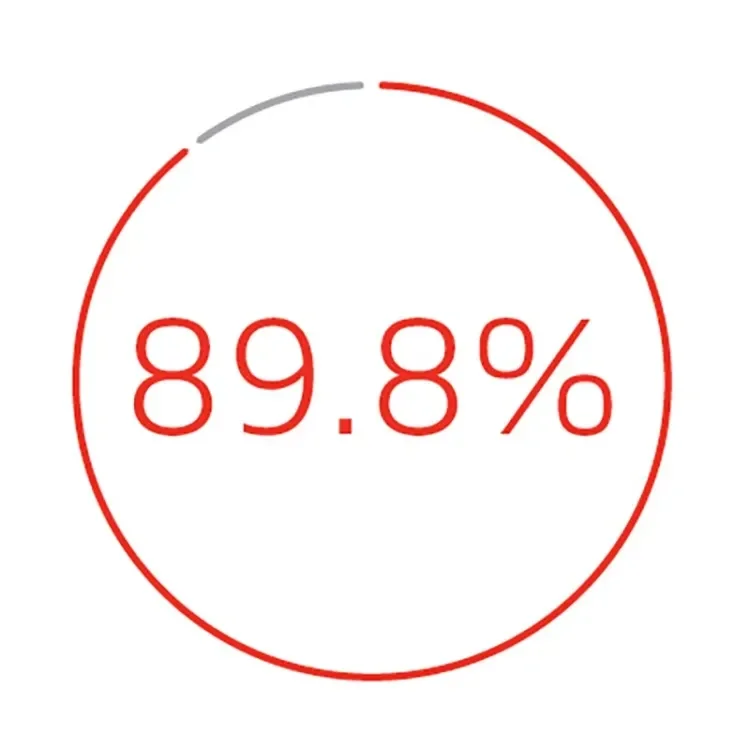
Primary patency at 42 months3
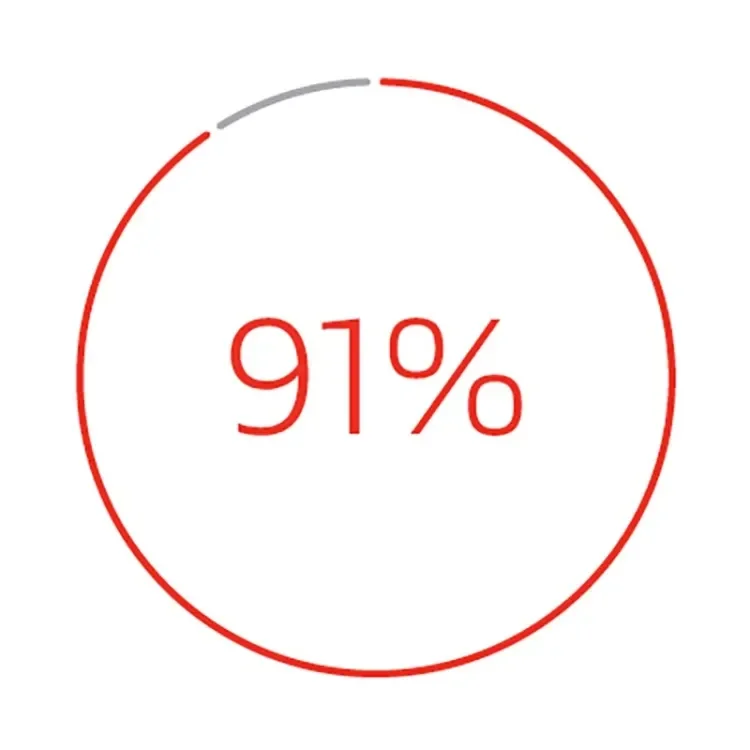
Secondary patency at 42 months3
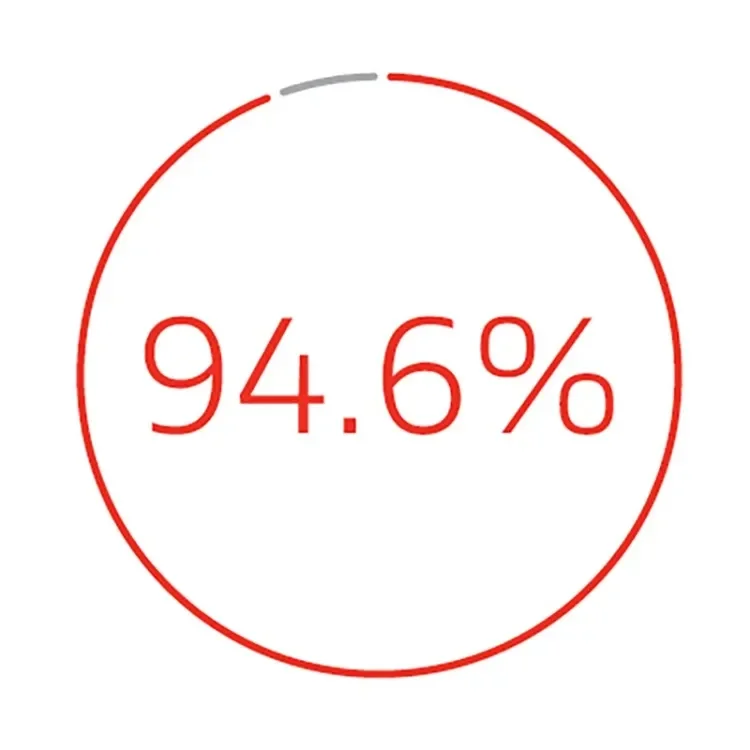
Limb salvage rate at 42 months3
* As used by Gore, PROPATEN Bioactive Surface refers to Gore’s proprietary CBAS® Heparin Surface.
† 59 subjects participated and 28 were available through the end of the study at 5-year follow-up.
‡ (P < .001) Statistically significant change from pre-procedure.
§ Complex defined as TASC II classification C & D lesions.
- Holden A, Takele E, Hill A, et al. Long-Term Follow-up of Subjects With Iliac Occlusive Disease Treated With the Viabahn VBX Balloon-Expandable Endoprosthesis. Journal of Endovascular Therapy. 2023;0(0). doi:10.1177/15266028231165723.
- Panneton JM, Bismuth J, Gray BH, Holden A. Three-year follow-up of patients with iliac occlusive disease treated with the Viabahn Balloon-Expandable Endoprosthesis. Journal of Endovascular Therapy 2020;27(5):728-736.
- Squizzato, F., Mosquera-Rey, V., Zanabili Al-Sibbai, A. et al. Outcomes of Self-Expanding Covered Stents for the Treatment of External ILIAC Artery Obstructive Disease. Cardiovasc Intervent Radiol 46, 579–587 (2023). https://doi.org/10.1007/s00270-023-03370-9

Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. RXOnly
CBAS is a trademark of Carmeda AB, a wholly owned subsidiary of W. L. Gore & Associates, Inc
24PL2118-EN01
