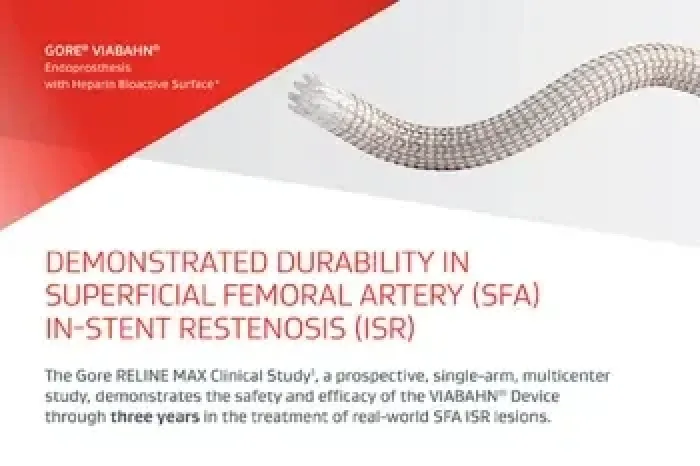
The GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface*,† for treating in-stent restenosis (ISR) of the superficial femoral artery (SFA)
Decide with data
Three-year study results from the Gore RELINE MAX Clinical Study demonstrate durable outcomes in the treatment of real-world SFA ISR lesions.1
These data were first presented during the late-breaking clinical trials session at VIVA 2022.
Investigator insights
See Peter Soukas, M.D., and Darren Schneider, M.D., discuss the objectives, outcomes and key implications of the 3-year data.
Gore RELINE MAX Clinical Study
Objective and methodology1
- Single-arm, prospective, multicenter study (23 U.S. sites and 3 European sites)
- 86 patients analyzed (Per-protocol)
- Real-world ISR lesion presentation:
- 52% long diffuse lesions
- 29% total occlusions
- 33% moderate/severe calcification
- 52% long diffuse lesions
PROVEN EFFICACY THROUGH 3 YEARS1:
65%
freedom from target lesion revascularization
>80%
of patients maintained ≥ 1 Rutherford category improvement
.24
improvement in mean resting ankle-brachial index (ABI)
(P < .001, .92 mean ABI)‡
PROVEN SAFETY THROUGH 3 YEARS1:
100%
freedom from major amputation§
100%
freedom from VIABAHN® Device stent fractures
Consider the complexity and challenges of treating SFA ISR:
- Case complexities such as calcification, total occlusions and long lesions have been associated with an increased rate of restenosis, occlusion and provisional stenting in SFA disease.2-7
- ISR is the “Achilles’ heel” of peripheral vascular interventions.8
- Physicians estimate 63% of ISR lesions are long and diffuse or total occlusions while 35% have a moderate to high degree of calcification.9
- Physicians estimate 63% of ISR lesions are long and diffuse or total occlusions while 35% have a moderate to high degree of calcification.9
- Reintervention frequency is cited as a top ISR treatment challenge, with physicians estimating the average patient experiences 7 ISR reinterventions over 3 years.9
Interested in more insights?
Hear more clinical considerations from the RELINE MAX study investigators.
TREATING SFA IN-STENT RESTENOSIS: CARL’S STORY
How the VIABAHN® Device improved Carl’s mobility and quality of life.
* As used by Gore, Heparin Bioactive Surface refers to Gore’s proprietary CBAS® Heparin Surface.
† Also referred to as the GORE® VIABAHN® Endoprosthesis with PROPATEN Bioactive Surface in some regions.
‡ (P < .001) Statistically significant change from preprocedure.
§ In a cohort including Rutherford category 4+ patients at baseline.
- Soukas P, Becker M, Stark K, Tepe G; RELINE MAX Investigators. Three-year results of the GORE® VIABAHN® endoprosthesis in the superficial femoral artery for in-stent restenosis. Journal of the Society for Cardiovascular Angiography & Interventions 2023;2(6)Part A:101183. OPEN ACCESS https://www.sciencedirect.com/science/article/pii/S2772930323011857
- Kum S. Evolution and challenges of ISR. How to approach. Presented at Leipzig Interventional Course (LINC 2020); January 28-31, 2020; Leipzig, Germany.
- Tepe G, Laird J, Schneider P, et al; IN.PACT SFA Trial Investigators. Drug-coated balloon versus standard percutaneous transluminal angioplasty for the treatment of superficial femoral and popliteal peripheral artery disease: 12-month results from the IN.PACT SFA Randomized Trial. Circulation 2015;131(5):495-502.
- Iida O, Takahara M, Soga Y, et al; ZEPHYR Investigators. 1-year results of the ZEPHYR Registry (Zilver PTX for the Femoral Artery and Proximal Popliteal Artery): predictors of restenosis. JACC: Cardiovascular Interventions 2015;8(8):1105-1112.
- Kistner CM, Lammer J, Willfort-Ehringer A, et al. Paclitaxel-eluting balloon versus standard balloon angioplasty in in-stent restenosis of the superficial femoral and proximal popliteal artery:1-year results of the PACUBA Trial. JACC: Cardiovascular Interventions 2016;9(13):1386-1392.
- Varghese V, Virk HUH, Lakhter V, et al. Femoral artery chronic total occlusion revascularization (FACTOR) score and algorithm: feasibility and validation in a single-center study of femoropopliteal occlusions. Journal of Invasive Cardiology 2020;32(12):E338-E348.
- Scheinert D, Micari A, Brodmann M, et al; IN.PACT Global Study Investigators. Drug-coated balloon treatment for femoropopliteal artery disease. Circulation:Cardiovascular Interventions 2018;11(10):e005654.
- Adams GL, Bersin RM, George JC, Subramanian V, Soukas PA. Data-driven treatment approach to in-stent restenosis. Endovascular Today 2015;14(6) Supplement:3-8.
- W. L. Gore & Associates Inc. Market Assessment for ISR in SFA Procedures- Global Research. Flagstaff, AZ: W. L. Gore & Associates, Inc; 2022. [Market research]. 22715502-EN.
- Bosiers M, Deloose K, Callaert J, et al. Stent-grafts are the best way to treat complex in-stent restenosis lesions in the superficial femoral artery: 24-month results from a multicenter randomized trial. The Journal of Cardiovascular Surgery 2020;61(5):617-25.

Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. RXOnly
INDICATIONS FOR USE IN THE U.S.: The GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface is indicated for improving blood flow in patients with symptomatic peripheral arterial disease in superficial femoral artery de novo and restenotic lesions up to 270 mm in length with reference vessel diameters ranging from 4.0 – 7.5 mm. The GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface is indicated for improving blood flow in patients with symptomatic peripheral arterial disease in superficial femoral artery in-stent restenotic lesions up to 270 mm in length with reference vessel diameters ranging from 4.0 – 6.5 mm. The GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface is indicated for improving blood flow in patients with symptomatic peripheral arterial disease in iliac artery lesions up to 80 mm in length with reference vessel diameters ranging from 4.0 – 12 mm. The GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface is also indicated for the treatment of stenosis or thrombotic occlusion at the venous anastomosis of synthetic arteriovenous (AV) access grafts.
CONTRAINDICATIONS: The GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface is contraindicated for non-compliant lesions where full expansion of an angioplasty balloon catheter was not achieved during pre-dilatation, or where lesions cannot be dilated sufficiently to allow passage of the delivery system. Do not use the GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface in patients with known hypersensitivity to heparin, including those patients who have had a previous incident of Heparin-Induced Thrombocytopenia (HIT) type II.