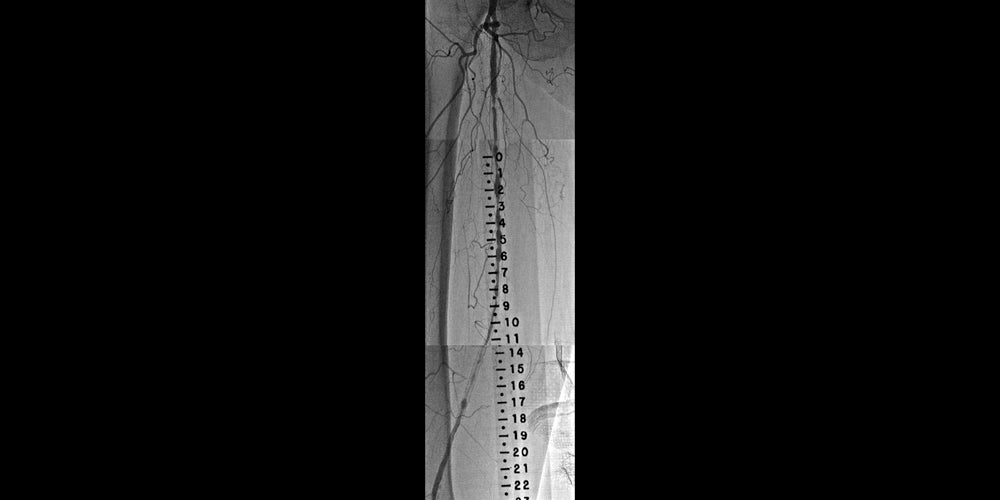
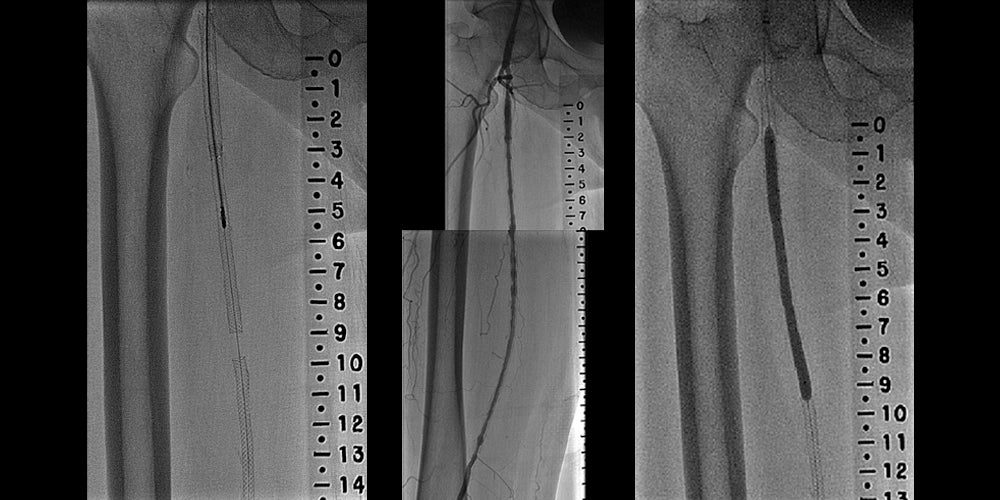
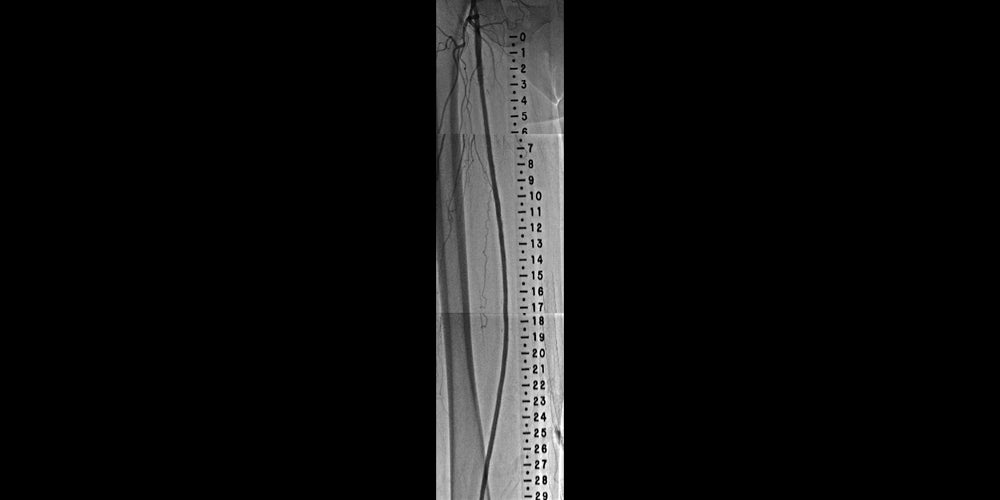
Diffuse in-stent restenosis (ISR) in long-stented segment in the superficial femoral artery (SFA)
Product indications and/or availability may vary by region. Please consult the product's Instructions for Use for your region for complete information, including indications, contraindications, warnings and precautions.
A case study using the GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface*,†
Challenge
- 59-year-old female with right calf claudication
- Relevant patient history:
- Diabetes, hypertension, hyperlipidemia, former smoker
- Previous bare-metal stenting of the right SFA for diffuse disease
- Recurrent severe right calf claudication six months later
Procedure
- Atherectomy with distal embolic protection was used to debulk the lesion
- Stent graft diameters (inflow and outflow) were selected based on the measured diameter of the healthy vessel proximal and distal to the bare-metal stents
- Stent grafts extended a minimum of 1 cm beyond bare-metal stents
Case Takeaways
- The use of the VIABAHN® Device provides an excellent treatment option for ISR in the SFA, particularly when it involves a long stented segment
- Atherectomy can be used as an adjunct to provide debulking of the restenotic tissue, and optimize stent graft expansion
Related case study
* As used by Gore, Heparin Bioactive Surface refers to Gore’s proprietary CBAS® Heparin Surface.
† Also referred to as the GORE® VIABAHN® Endoprosthesis with PROPATEN Bioactive Surface in some regions.
The outcomes and observations reported are based on individual case experience and the patients treated. The steps described here may not be complete, and are not intended to be a replacement for the Instructions for Use (IFU) or the education, training and professional judgment of health care providers (HCP). HCPs remain solely responsible for making decisions about patient care and the use of medical technologies.

Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. RXOnly
INDICATIONS FOR USE IN THE U.S.: The GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface is indicated for improving blood flow in patients with symptomatic peripheral arterial disease in superficial femoral artery de novo and restenotic lesions up to 270 mm in length with reference vessel diameters ranging from 4.0 – 7.5 mm. The GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface is indicated for improving blood flow in patients with symptomatic peripheral arterial disease in superficial femoral artery in-stent restenotic lesions up to 270 mm in length with reference vessel diameters ranging from 4.0 – 6.5 mm. The GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface is indicated for improving blood flow in patients with symptomatic peripheral arterial disease in iliac artery lesions up to 80 mm in length with reference vessel diameters ranging from 4.0 – 12 mm. The GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface is also indicated for the treatment of stenosis or thrombotic occlusion at the venous anastomosis of synthetic arteriovenous (AV) access grafts.
CONTRAINDICATIONS: The GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface is contraindicated for non-compliant lesions where full expansion of an angioplasty balloon catheter was not achieved during pre-dilatation, or where lesions cannot be dilated sufficiently to allow passage of the delivery system. Do not use the GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface in patients with known hypersensitivity to heparin, including those patients who have had a previous incident of Heparin-Induced Thrombocytopenia (HIT) type II.