Reline using the GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface*,† as first line therapy when treating complex in-stent restenosis (ISR)
Product indications and/or availability may vary by region. Please consult the product's Instructions for Use for your region for complete information, including indications, contraindications, warnings and precautions.
A case study using the VIABAHN® Device
Challenge
- In 2007 (age 72) the patient presented with claudication requiring superficial femoral artery (SFA) cryoplasty
- Over an 11-year period the patient received multiple reinterventions:
- Less than three months after initial treatment — Symptoms returned. Patient was treated with laser atherectomy at adductor hiatus and a 7 x 100 mm self-expanding bare metal nitinol stent was placed.
- 2011 — Recurrent symptoms due to ISR treated with laser atherectomy and plain old balloon angioplasty (POBA)
- 2012 — Repeat laser atherectomy and POBA
- 2015 — Debilitating dyspnea and return of distal disease based on drop of ankle-brachial index and elevated duplex velocity with Ratio > 2; No peripheral intervention undertaken as limb ischemia was less symptomatic than coronary disease
- 2018 (age 83) — Successful transcatheter aortic valve replacement and alleviation of dyspnea. Patient re-presented with significant short distance claudication with inability to complete cardiac rehab; ISR with peak systolic velocity > 400 cm / s
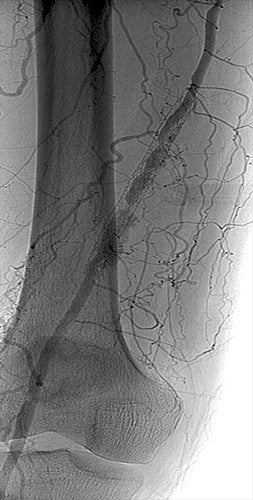
Image courtesy of Benjamin J. Pearce, M.D. Used with permission.
Procedure
- Due to multiple attempts at prior atherectomy and POBA, procedure entered with intent to reline the bare metal stents (BMS) with the VIABAHN® Device
- Contralateral access gained to perform aortogram and rule out inflow / outflow disease
- A 6 Fr TERUMO® DESTINATION® Guiding Sheath placed after anticoagulation
- Angio confirmed ISR with two vessel runoff via large anterior tibial and peroneal arteries and some proximal progression of SFA disease
- Crossed with 0.035” SPECTRANETICS QUICK-CROSS® Support Catheter and TERUMO® GLIDEWIRE® Guidewire
- A 7 mm MEDTRONIC SPIDERFX Embolic Protection Device placed through SPECTRANETICS QUICK-CROSS® Support Catheter
- Pre-treated with a 5 x 100 mm POBA including new disease proximal to old stent
- First deployed a 6 mm x 15 cm VIABAHN® Device and then overlapped with a 6 mm x 10 cm VIABAHN® Device
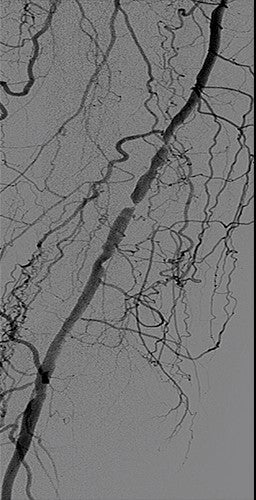
Image courtesy of Benjamin J. Pearce, M.D. Used with permission.
Result
- Completion with no significant residual stenosis and no embolization of runoff
- Due to age and travel, the patient was kept overnight and discharged the next morning with palp dorsalis pedis pulse
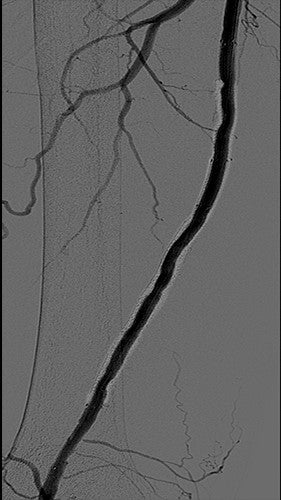
Image courtesy of Benjamin J. Pearce, M.D. Used with permission.
Case Takeaways
For any complex SFA ISR, relining with covered stent grafts has become a “go to” procedure as first line therapy. The Gore RELINE Clinical Study with the VIABAHN® Device showed high primary patency even in the most challenging disease: 75% 12-month primary patency with an average lesion length of over 17 cm.1 In this ISR disease case, relining the BMS with a VIABAHN® Device produced a favorable outcome and successfully excluded the neointimal hyperplasia associated with ISR of the BMS.
Related case study
TERUMO, DESTINATION and GLIDEWIRE are trademarks of Terumo Medical Corporation.
SPECTRANETICS and QUICK-CROSS are trademarks of Spectranetics Corp.
MEDTRONIC and SPIDERFX are trademarks of Medtronic, Inc.
The outcomes and observations reported are based on individual case experience and the patients treated. The steps described here may not be complete, and are not intended to be a replacement for the Instructions for Use (IFU) or the education, training and professional judgment of health care providers (HCP). HCPs remain solely responsible for making decisions about patient care and the use of medical technologies.
* As used by Gore, Heparin Bioactive Surface refers to Gore's proprietary CBAS® Heparin Surface.
† Also referred to as the GORE® VIABAHN® Endoprosthesis with PROPATEN Bioactive Surface in some regions.
1. Bosiers M, Deloose K, Callaert J, et al. Superiority of stent-grafts for in-stent restenosis in the superficial femoral artery: twelve-month results from a multicenter randomized trial. Journal of Endovascular Therapy 2015;22(1):1-10.

Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. RXOnly
INDICATIONS FOR USE IN THE U.S.: The GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface is indicated for improving blood flow in patients with symptomatic peripheral arterial disease in superficial femoral artery de novo and restenotic lesions up to 270 mm in length with reference vessel diameters ranging from 4.0 – 7.5 mm. The GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface is indicated for improving blood flow in patients with symptomatic peripheral arterial disease in superficial femoral artery in-stent restenotic lesions up to 270 mm in length with reference vessel diameters ranging from 4.0 – 6.5 mm. The GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface is indicated for improving blood flow in patients with symptomatic peripheral arterial disease in iliac artery lesions up to 80 mm in length with reference vessel diameters ranging from 4.0 – 12 mm. The GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface is also indicated for the treatment of stenosis or thrombotic occlusion at the venous anastomosis of synthetic arteriovenous (AV) access grafts.
CONTRAINDICATIONS: The GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface is contraindicated for non-compliant lesions where full expansion of an angioplasty balloon catheter was not achieved during pre-dilatation, or where lesions cannot be dilated sufficiently to allow passage of the delivery system. Do not use the GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface in patients with known hypersensitivity to heparin, including those patients who have had a previous incident of Heparin-Induced Thrombocytopenia (HIT) type II.