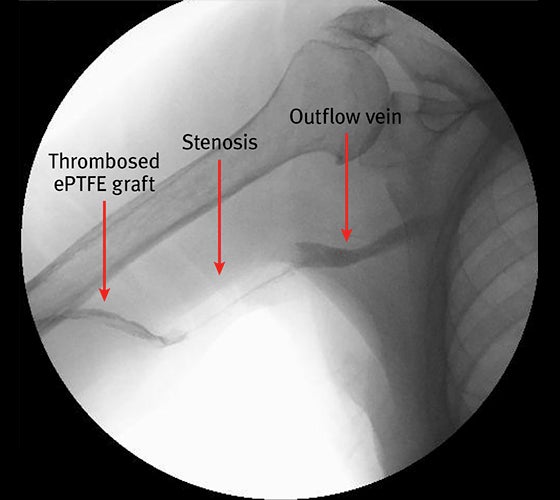
Management of vascular graft stenosis in a patient with recurrent thrombosis
GORE® VIABAHN® Endoprosthesis with PROPATEN Bioactive Surface is not authorized for the treatment of stenosis or thrombotic occlusion at the venous anastomosis of synthetic arteriovenous (AV) access grafts in Canada.
A case study using the GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface*,†
Challenge
- Right brachial artery to axillary vein ePTFE was placed but developed a venous anastomosis stenosis with thrombosis of the graft at 13 months post creation (see image to the left)
- Relevant patient history:
- 84-year-old end-stage renal disease patient with a history of recurrent thrombosis and stenosis of ePTFE grafts
- Left arm graft was dialyzed for 23 months before it was abandoned after three episodes in two months of recurrent thrombosis attributed to venous anastomosis stenosis and managed by balloon angioplasty
Procedure
- An endovascular thrombectomy of the right arm graft was performed. Antegrade and retrograde access was obtained directly through the ePTFE graft
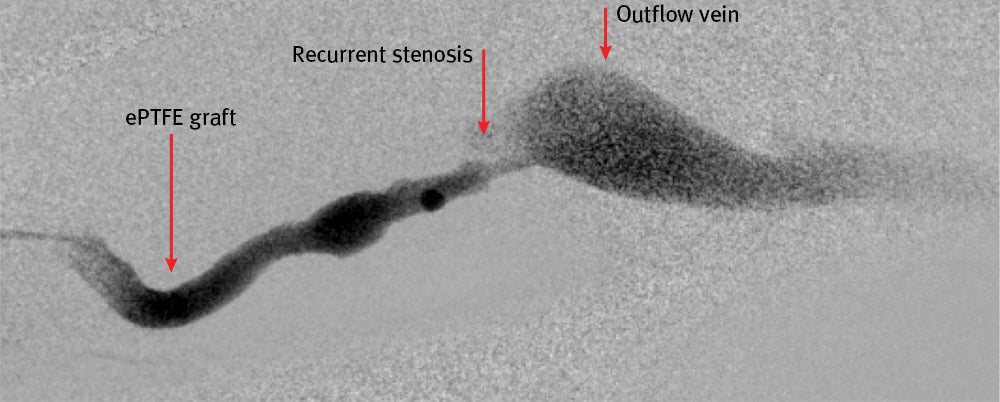
- A pullback angiogram confirmed venous anastomosis stenosis, resulting in graft thrombosis
- Heparin was administered and angioplasty performed. Further maceration of the graft thrombus, EDWARDS FOGARTY® Catheter clearance of the arterial plug, and clot removal with thromboaspiration was performed.
- With flow restored to the graft, the graft remained pulsatile with a weak thrill
- Stenosis was persistent and observed at the graft venous anastomosis
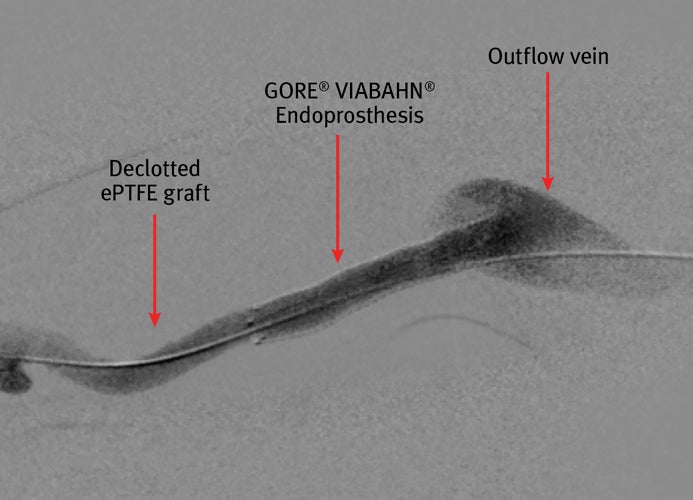
- A 8mm x 5 cm VIABAHN® Device was placed at the venous anastomosis resulting in restoration of brisk flow and strong thrill in access, with resolution of recoil
Images courtesy of Daniel V. Patel, M.D. Used with permission.
Result
- At 60-months postplacement, the VIABAHN® Device has maintained secondary patency without any further episodes of thrombosis
- The patient has required periodic intra-graft angioplasty on average two to three times per year
Images courtesy of Daniel V. Patel, M.D. Used with permission.
Case Takeaways
- In this case, balloon angioplasty management of the previous left arm graft ultimately resulted in graft failure and recurrent thrombosis at just under two years
- At six years the right arm graft has maintained significantly longer secondary patency than the original left arm graft, with no further episodes of graft thrombosis after placement of the VIABAHN® Device at the venous anastomosis
- The VIABAHN® Device is an excellent option for management of the venous anastomosis of AV grafts versus balloon angioplasty, as demonstrated in the Gore REVISE Clinical Study1
Related case study
EDWARDS and FOGARTY® are trademarks of Edwards Lifesciences Corporation.
The outcomes and observations reported are based on individual case experience and the patients treated. The steps described here may not be complete, and are not intended to be a replacement for the Instructions for Use (IFU) or the education, training and professional judgment of health care providers (HCP). HCPs remain solely responsible for making decisions about patient care and the use of medical technologies.
* As used by Gore, Heparin Bioactive Surface refers to Gore's proprietary CBAS® Heparin Surface.
† Also referred to as the GORE® VIABAHN® Endoprosthesis with PROPATEN Bioactive Surface in some regions.
1. Vesely T, DaVanzo W, Behrend T, Dwyer A, Aruny J. Balloon angioplasty versus Viabahn stent graft for treatment of failing or thrombosed prosthetic hemodialysis grafts. Journal of Vascular Surgery 2016; 64(5):1400-1410.e1. http://www.sciencedirect.com/science/article/pii/S0741521416301756
2. Mohr BA, Sheen AL, Roy-Chaudhury P, Schultz SR, Aruny JE; REVISE Investigators. Clinical and economic benefits of stent grafts in dysfunctional and thrombosed hemodialysis access graft circuits in the REVISE Randomized Trial. Journal of Vascular & Interventional Radiology 2018;30(2):203-211.e4.

Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. RXOnly
INDICATIONS FOR USE IN THE U.S.: The GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface is indicated for improving blood flow in patients with symptomatic peripheral arterial disease in superficial femoral artery de novo and restenotic lesions up to 270 mm in length with reference vessel diameters ranging from 4.0 – 7.5 mm. The GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface is indicated for improving blood flow in patients with symptomatic peripheral arterial disease in superficial femoral artery in-stent restenotic lesions up to 270 mm in length with reference vessel diameters ranging from 4.0 – 6.5 mm. The GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface is indicated for improving blood flow in patients with symptomatic peripheral arterial disease in iliac artery lesions up to 80 mm in length with reference vessel diameters ranging from 4.0 – 12 mm. The GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface is also indicated for the treatment of stenosis or thrombotic occlusion at the venous anastomosis of synthetic arteriovenous (AV) access grafts.
CONTRAINDICATIONS: The GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface is contraindicated for non-compliant lesions where full expansion of an angioplasty balloon catheter was not achieved during pre-dilatation, or where lesions cannot be dilated sufficiently to allow passage of the delivery system. Do not use the GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface in patients with known hypersensitivity to heparin, including those patients who have had a previous incident of Heparin-Induced Thrombocytopenia (HIT) type II.
INDICATIONS FOR USE IN CANADA: The GORE® VIABAHN® Endoprosthesis with PROPATEN Bioactive Surface is a flexible, self-expanding endoluminal prosthesis for endovascular grafting of peripheral arteries.
CONTRAINDICATIONS: The GORE® VIABAHN® Endoprosthesis with PROPATEN Bioactive Surface is contraindicated for non-compliant lesions where full expansion of an angioplasty balloon catheter was not achieved during pre-dilatation, or where lesions cannot be dilated sufficiently to allow passage of the delivery system. Do not use the GORE® VIABAHN® Endoprosthesis with PROPATEN Bioactive Surface in patients with known hypersensitivity to heparin, including those patients who have had a previous incident of Heparin-Induced Thrombocytopenia (HIT) type II.