Treating claudication and rest pain due to chronic total occlusion of the SFA
A case study using the GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface*,†
Procedure
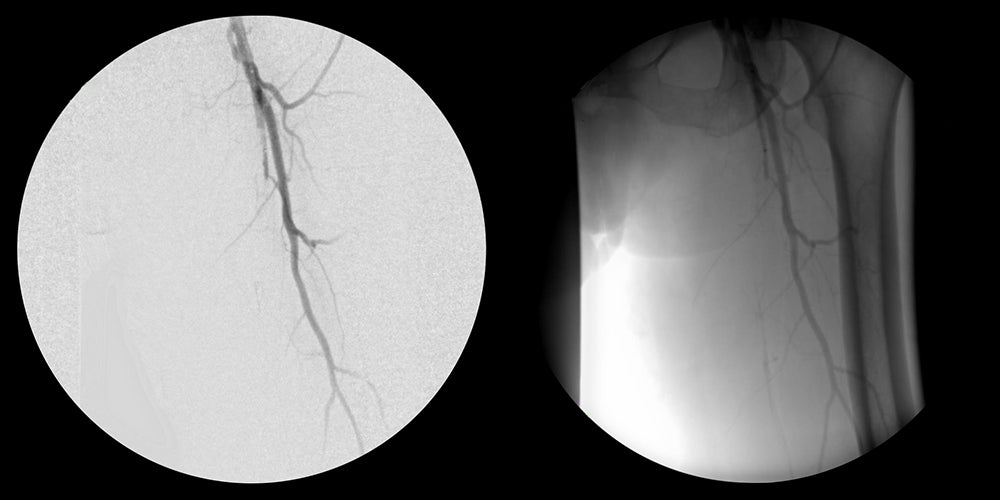
- Obtained percutaneous access into the right femoral artery with ultrasound guidance. Then performed an angiogram of the left lower extremity.
- Crossed SFA chronic total occlusion (CTO) with 035 TERUMO® RADIOFOCUS® GLIDEWIRE® ADVANTAGE and 035 TERUMO® NAVICROSS® Support Catheter
- Atherectomy completed with CARDIOVASCULAR SYSTEMS DIAMONDBACK 360® Peripheral Orbital Atherectomy System 2.0 Solid Crown
- Followed by angioplasty with a 5 mm percutaneous transluminal angioplasty (PTA) balloon
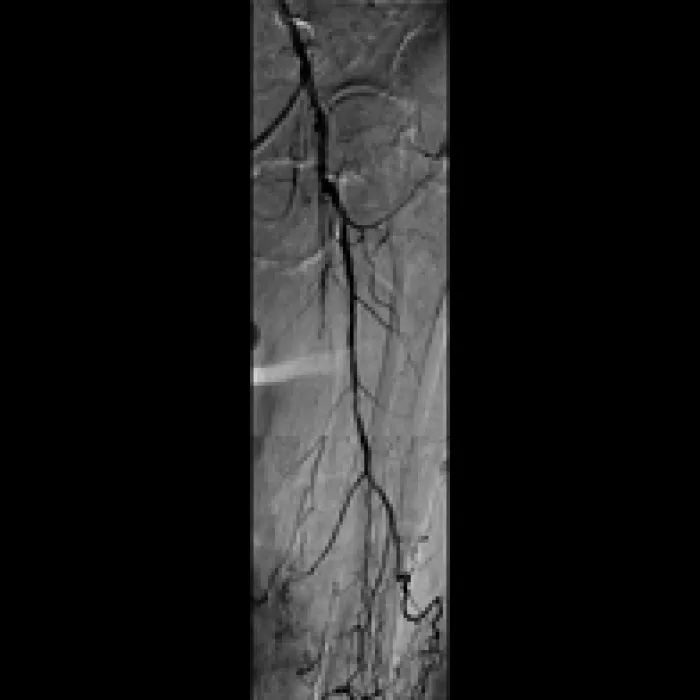
- Deployed two 6 mm x 15 cm VIABAHN® Devices in the SFA and post-dilated with 6 mm PTA balloon
Result
- Completion angiogram showed excellent flow of the left lower extremity. Immediately post-op, complete resolution of left sided severe claudication.
- At follow-up office visit 2.5 weeks post-op, the patient presented with complete resolution of claudication and rest pain. Patient had palpable tibial pulses bilaterally
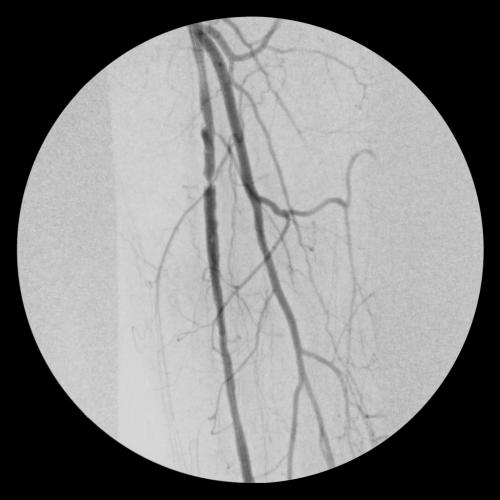
Images courtesy of James Otto, M.D. Used with permission.
Case Takeaways
As demonstrated in this case, the VIABAHN® Device offers excellent patency in the treatment of long SFA total occlusions and should be considered first-line treatment for complex SFA disease.
The VIABAHN® Device has demonstrated durability and long-term patency in treatment of complex SFA disease with
97% three-year secondary patency
(27 cm average lesion length, 93% CTOs)1
Related case study
* As used by Gore, Heparin Bioactive Surface refers to Gore's proprietary CBAS® Heparin Surface.
† Also referred to as the GORE® VIABAHN® Endoprosthesis with PROPATEN Bioactive Surface in some regions.
1. Böhme T, Noory E, Brechtel K, et al. Heparin-bonded stent-graft for the treatment of TASC II C and D femoropopliteal lesions: 36-month results of the Viabahn 25 cm trial. Journal of Endovascular Therapy. 2021;28(2) 222-228. https://journals.sagepub.com/doi/pdf/10.1177/1526602820965965
This content is for informational purposes only, is not advice or a guarantee of outcome. It is not a substitute for professional medical advice, diagnosis or treatment. Individual results and/or treatment may vary based upon the circumstances, the patient’s specific situation, and the healthcare provider’s medical judgment.

Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. RXOnly
INDICATIONS FOR USE IN THE U.S.: The GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface is indicated for improving blood flow in patients with symptomatic peripheral arterial disease in superficial femoral artery de novo and restenotic lesions up to 270 mm in length with reference vessel diameters ranging from 4.0 – 7.5 mm. The GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface is indicated for improving blood flow in patients with symptomatic peripheral arterial disease in superficial femoral artery in-stent restenotic lesions up to 270 mm in length with reference vessel diameters ranging from 4.0 – 6.5 mm. The GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface is indicated for improving blood flow in patients with symptomatic peripheral arterial disease in iliac artery lesions up to 80 mm in length with reference vessel diameters ranging from 4.0 – 12 mm. The GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface is also indicated for the treatment of stenosis or thrombotic occlusion at the venous anastomosis of synthetic arteriovenous (AV) access grafts.
CONTRAINDICATIONS: The GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface is contraindicated for non-compliant lesions where full expansion of an angioplasty balloon catheter was not achieved during pre-dilatation, or where lesions cannot be dilated sufficiently to allow passage of the delivery system. Do not use the GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface in patients with known hypersensitivity to heparin, including those patients who have had a previous incident of Heparin-Induced Thrombocytopenia (HIT) type II.