Consider the complexity of treating chronic total occlusions (CTOs)
- CTOs can increase the need for provisional stenting.1
- In the Medtronic IN.PACT Global Study, treatment of CTOs increased the rate of provisional stenting by 85%.
- Although a rare complication, the risk of distal embolization has been shown to increase when treating a CTO.2-3
- Limited comparison data exists between newer endovascular technologies compared to surgical bypass, the current recommended treatment for CTOs.4
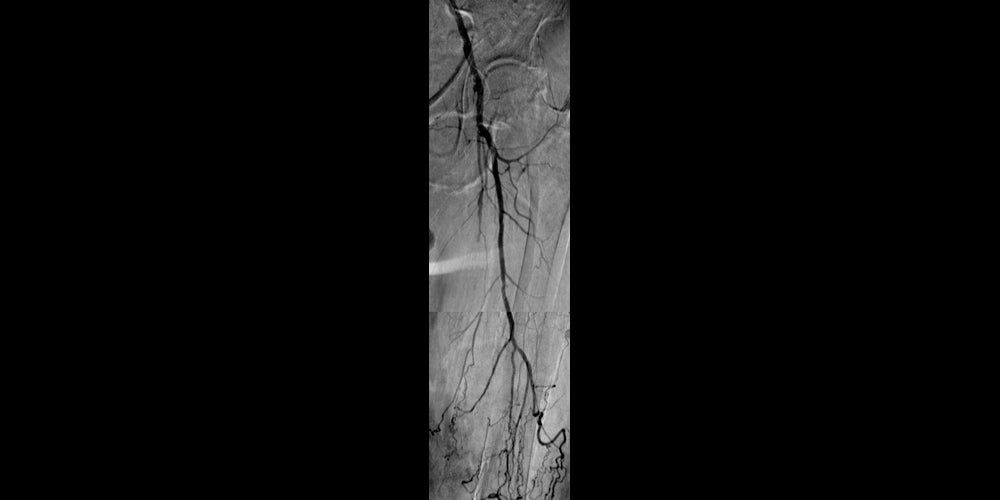
Before
Left SFA CTO with one-vessel runoff (not shown).
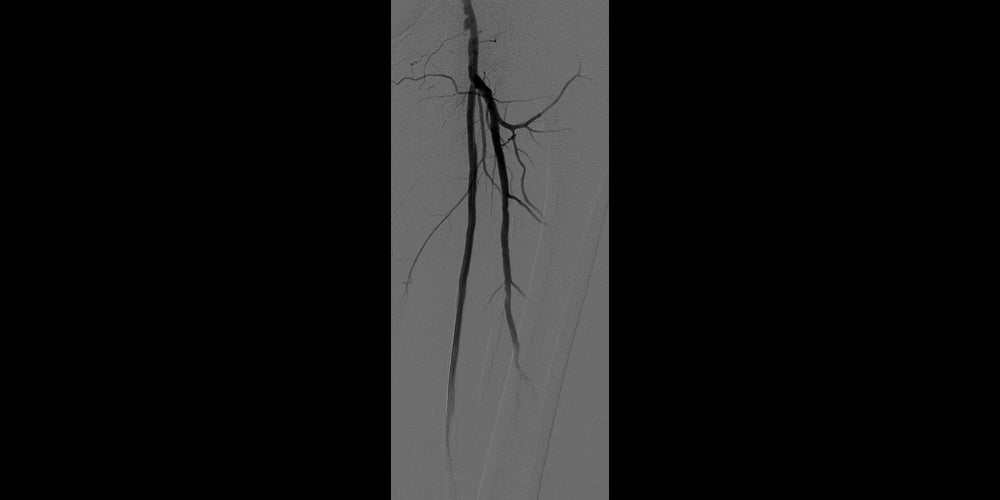
After
Post-placement of two 6 mm x 15 cm GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface*,†. Maintained one-vessel runoff. (not shown)
The VIABAHN® Device has been extensively studied in CTOs5-9
Proven patency
72% of limbs studied in 5 multicenter, prospective, randomized or single arm studies were CTOs (302 / 422) that achieved
75% primary patency5-9
Demonstrated durability
97%
3-year secondary patency in complex disease
(27 cm average lesion length, 93% CTOs).10
Comparable clinical results to above knee surgical bypass (both prosthetic11 and native vein5).
The VIABAHN® Device design is uniquely capable of treating CTOs
- Expanded PTFE (ePTFE) flow lumen covers and seals off residual thrombosis.
- CBAS® Heparin Surface, also featured in the GORE® PROPATEN® Vascular Graft, is the proven lasting heparin bonding technology designed to resist thrombus formation.12
* As used by Gore, Heparin Bioactive Surface refers to Gore’s proprietary CBAS® Heparin Surface.
† Also referred to as the GORE® VIABAHN® Endoprosthesis with PROPATEN Bioactive Surface in some regions.
1. Brodmann M. Real world value of the In.Pact Admiral DCB (Medtronic) for fem-pop lesions: from the In.Pact Global Registry: what else does it tell us. Presented at the 44th Annual Symposium on Vascular and Endovascular Issues, Techniques, Horizons (VEITH symposium); November 14-18, 2017; New York, NY.
2. Wu W, Hua S, Li Y, et al. Incidence, risk factors, treatment and prognosis of popliteal artery embolization in the superficial femoral artery interventions. PLoS One 2014;9(9):e107717.
3. Shrikhande GV, Khan SZ, Hussain HG, Dayal R, McKinsey JF, Morrissey N. Lesion types and device characteristics that predict distal embolization during percutaneous lower extremity interventions. Journal of Vascular Surgery 2011;53(2):347-352.
4. Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG; TASC II Working Group. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). Journal of Vascular Surgery 2007;45(1)Supplement:S5-S67.
5. Reijnen M, van Walraven L, Fritschy W, et al. 1-year results of a multicenter, randomized controlled trial comparing heparin-bonded endoluminal to femoropopliteal bypass. Journal of Cardiovascular Interventions 2017;10(22):2320-2331.
6. Ohki T, Kichikawa K, Yokoi H, et al. Outcomes of the Japanese multicenter Viabahn trial of endovascular stent grafting for superficial femoral artery lesions. Journal of Vascular Surgery 2017;66(1):130-142.e1.
7. Saxon RR, Chervu A, Jones PA, et al. Heparin-bonded, expanded polytetrafluoroethylene-lined stent graft in the treatment of femoropopliteal artery disease: 1year results of the VIPER (Viabahn Endoprosthesis with Heparin Bioactive Surface in the Treatment of Superficial Femoral Artery Obstructive Disease) Trial. Journal of Vascular & Interventional Radiology 2013;24(2):165-173.
8. Lammer J, Zeller T, Hausegger KA, et al. Sustained benefit at 2 years for covered stents versus bare-metal stents in long SFA lesions: the VIASTAR trial. Cardiovascular & Interventional Radiology 2015;38(1):25-32.
9. Zeller T, Peeters P, Bosiers M, et al. Heparin-bonded stent-graft for the treatment of TASC II C and D femoropopliteal lesions: the Viabahn25 cm Trial. Journal of Endovascular Therapy 2014;21(6):765774.
10. GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface Instructions for Use (IFU). W. L. Gore & Associates, Inc. Accessed February 8, 2023. https://eifu.goremedical.com
11. McQuade K, Gable D, Pearl G, Theune B, Black S. Four-year randomized prospective comparison of percutaneous ePTFE/nitinol self-expanding stent graft versus prosthetic femoral-popliteal bypass in the treatment of superficial femoral artery occlusive disease. Journal of Vascular Surgery 2010;52(3):584-591.
12. CBAS® Heparin Surface. References. W. L. Gore & Associates, Inc. Accessed March 24, 2023. https://www.goremedical.com/cbas/references

Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. RXOnly
INDICATIONS FOR USE IN THE U.S.: The GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface is indicated for improving blood flow in patients with symptomatic peripheral arterial disease in superficial femoral artery de novo and restenotic lesions up to 270 mm in length with reference vessel diameters ranging from 4.0 – 7.5 mm. The GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface is indicated for improving blood flow in patients with symptomatic peripheral arterial disease in superficial femoral artery in-stent restenotic lesions up to 270 mm in length with reference vessel diameters ranging from 4.0 – 6.5 mm. The GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface is indicated for improving blood flow in patients with symptomatic peripheral arterial disease in iliac artery lesions up to 80 mm in length with reference vessel diameters ranging from 4.0 – 12 mm. The GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface is also indicated for the treatment of stenosis or thrombotic occlusion at the venous anastomosis of synthetic arteriovenous (AV) access grafts.
CONTRAINDICATIONS: The GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface is contraindicated for non-compliant lesions where full expansion of an angioplasty balloon catheter was not achieved during pre-dilatation, or where lesions cannot be dilated sufficiently to allow passage of the delivery system. Do not use the GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface in patients with known hypersensitivity to heparin, including those patients who have had a previous incident of Heparin-Induced Thrombocytopenia (HIT) type II.