GORE® SEAMGUARD® Bioabsorbable Staple Line Reinforcement Data Review: Leaks
Protects against leaks
The only* staple line reinforcement proven to significantly reduce leaks in sleeve gastrectomy procedures.1
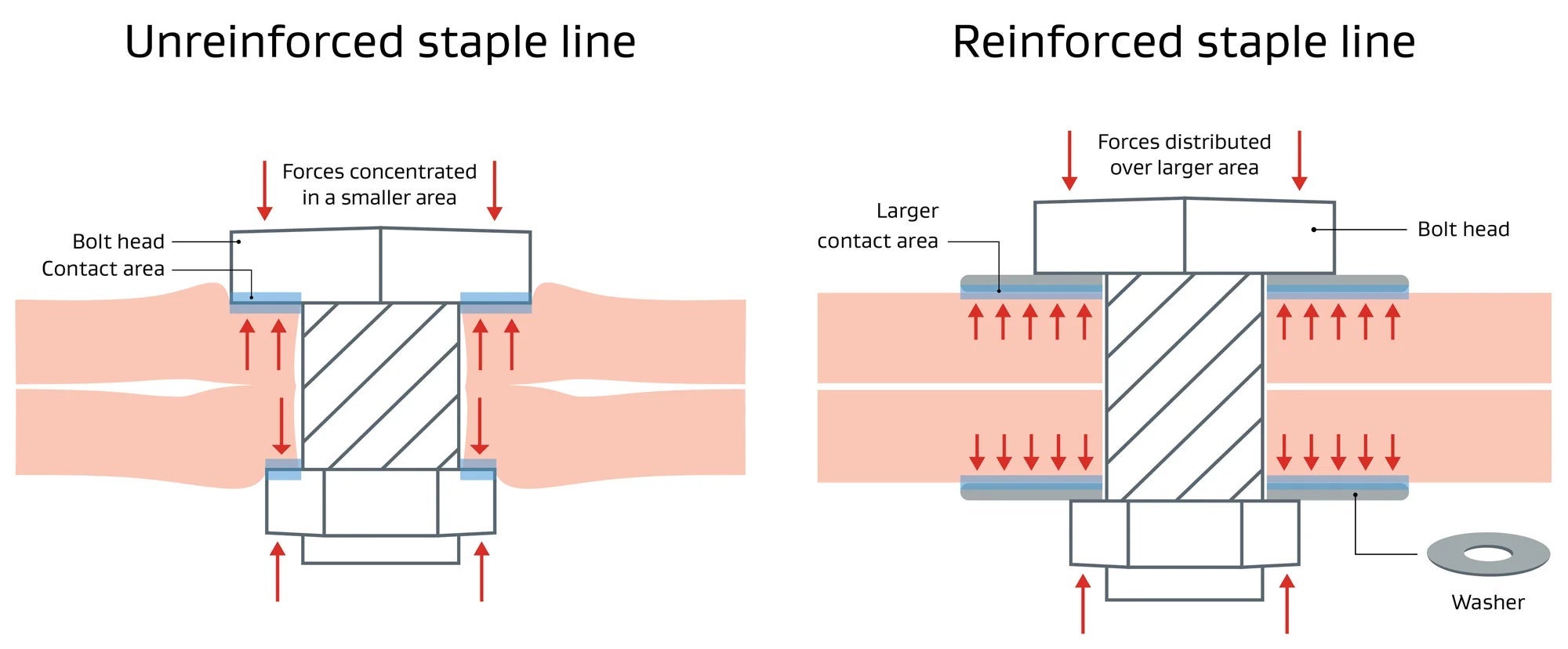
Pressure =
Force
Area
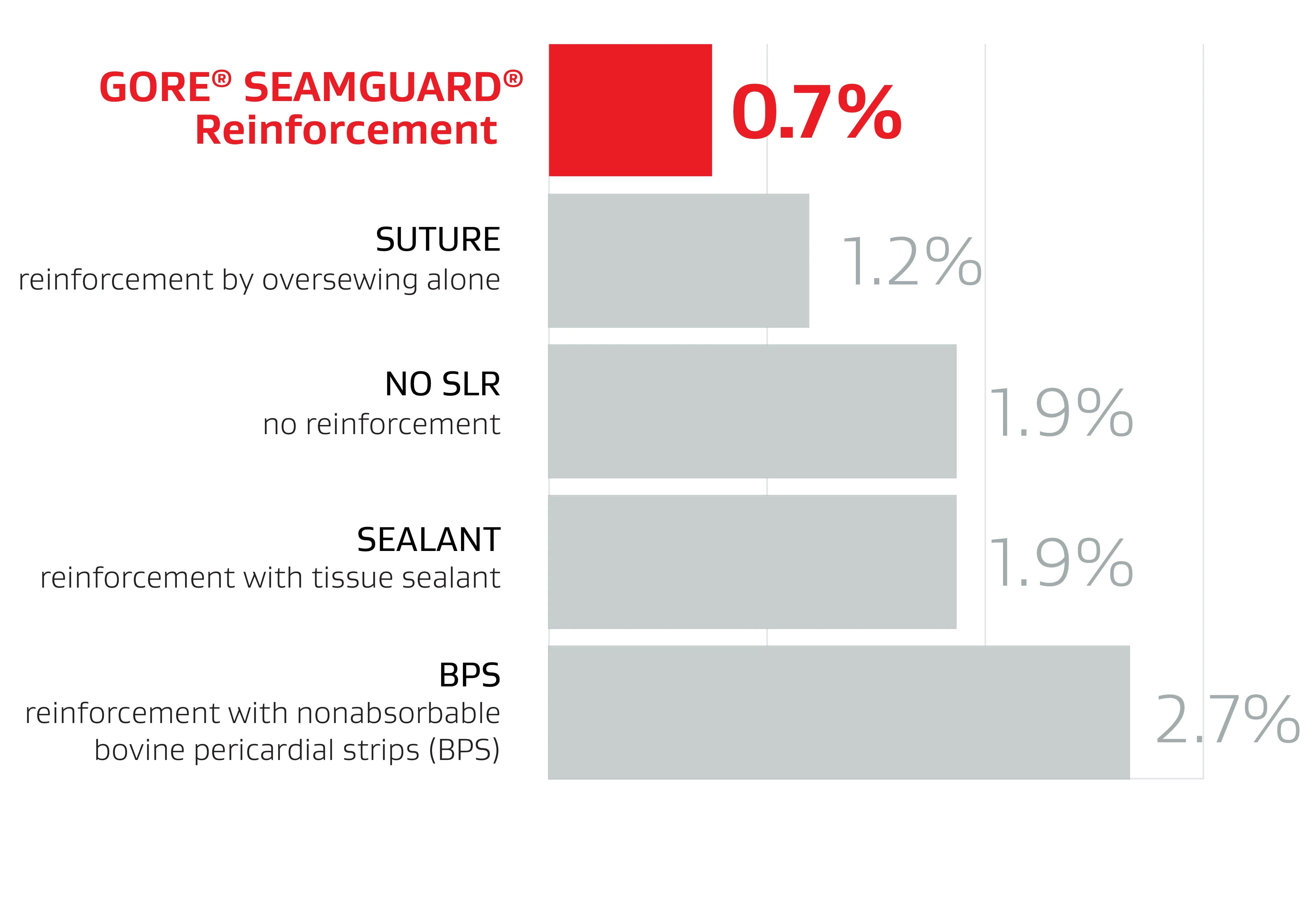
GORE® SEAMGUARD® Reinforcement had a significantly lower leak rate compared to other methods1
148 papers included in analysis
40,653 patients
* Considering all systematic review and meta-analysis of published articles only that distinguish between types of staple line reinforcement.
- Gagner M, Kemmeter P. Comparison of laparoscopic sleeve gastrectomy leak rates in five staple-line reinforcement options: a systematic review. Surgical Endoscopy 2020;34(1):396-407.
- McCrea C. GORE® SEAMGUARD® Reinforcement Product Family: Relationship between Design Inputs and “Engineered to reduce the incidence of perioperative leaks and bleeding” Marketing Statement. Flagstaff, AZ; 2015. [Work plan]. WP107241

Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. RXOnly
CONFIGURED FOR ENDOSCOPIC SURGICAL STAPLERS:
INDICATIONS FOR USE: GORE® SEAMGUARD® Bioabsorbable Staple Line Reinforcement is indicated for use in surgical procedures in which soft tissue transection or resection with staple line reinforcement is needed. GORE® SEAMGUARD® Bioabsorbable Staple Line Reinforcement can be used for reinforcement of staple lines during lung resection, bronchial, bariatric, colon, colorectal, gastric, mesentery, pancreas and small bowel procedures.
CONTRAINDICATIONS: Not for the patch reconstruction of cardiovascular defects such as cardiac, great vessel and peripheral vascular arteries or veins.
CONFIGURED FOR CIRCULAR SURGICAL STAPLERS:
INDICATIONS FOR USE: GORE® SEAMGUARD® Bioabsorbable Staple Line Reinforcement is indicated for use in surgical procedures in which a soft tissue anastomosis with staple line reinforcement is needed. GORE® SEAMGUARD® Bioabsorbable Staple Line Reinforcement can be used for reinforcement of staple lines during bariatric, colon, colorectal, gastric and small bowel procedures.
CONTRAINDICATIONS: Not for the reconstruction of cardiovascular defects such as cardiac, great vessel and peripheral vascular arteries or veins.
CONFIGURED FOR ROBOTIC ENDOSCOPIC SURGICAL STAPLERS:
INDICATIONS FOR USE: GORE® SEAMGUARD® Bioabsorbable Staple Line Reinforcement is indicated for use in surgical procedures in which soft tissue transection or resection with staple line reinforcement is needed. GORE® SEAMGUARD® Bioabsorbable Staple Line Reinforcement can be used for reinforcement of staple lines during lung resection, liver resection, bronchial, bariatric, colon, colorectal, esophagus, gastric, mesentery, pancreas, small bowel and spleen procedures.
GORE® SEAMGUARD® Bioabsorbable Staple Line Reinforcement is also intended to be used for reinforcement of suture-lines and staple-lines (i.e., occlusion of the left atrial appendage during open chest procedures) during cardiac surgery.
CONTRAINDICATIONS: Not for the patch reconstruction of cardiovascular defects such as cardiac, great vessel and peripheral vascular arteries or veins.
GORE® SEAMGUARD® Bioabsorbable Staple Line Reinforcement Configured for Robotic Endoscopic Surgical Staplers is not authorized for use in Canada.