
Minimize perioperative leaks and bleeding in staple line formation
GORE® SEAMGUARD® Reinforcement has achieved an unrivaled legacy of clinical performance,* impacting millions of patients† for more than 20 years.‡
Unrivaled legacy of clinical performance*
Millions of patients impacted†
85+ clinical studies
published and peer-reviewed§
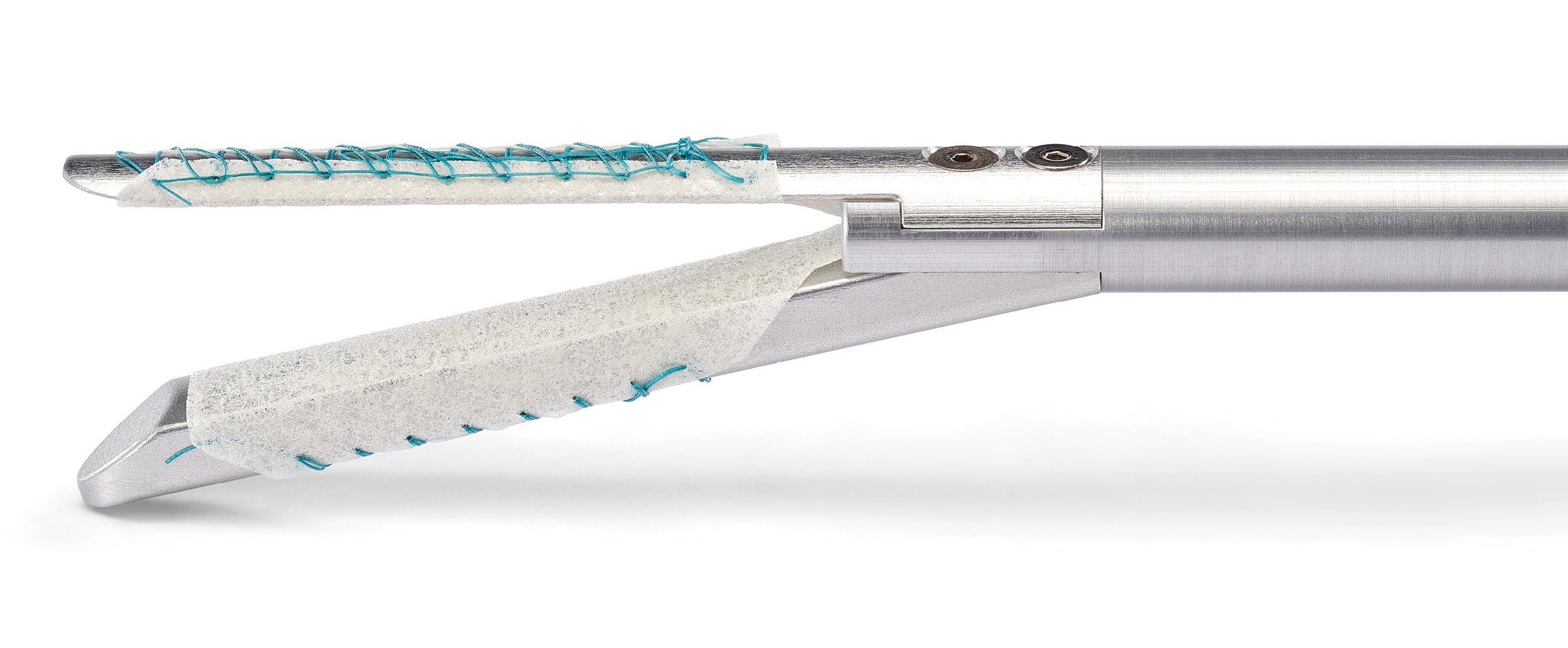
Compatible with robotic procedures||
Buttressing on robotic staplers
Erik B. Wilson, M.D., FACS discusses the concept of using staple line reinforcement with robotic staplers and explains how the buttress redistributes pressure along the staple line and how this compares to the mechanism of action and measurements of the robotic stapler.
Simplify inventory
Designed to work with a wide range of staplers, including robotic staplers||
Reduce overall postoperative complication costs
Provides reliable protection from surgical complications associated with leaks and bleeding, along with reduced reoperations and readmissions1-6
Leak rate
Less than 0.39% in the U.S. (0.7 % overall.)2
Protects against bleeding
Randomized prospective data has shown that the GORE® SEAMGUARD® Reinforcement significantly reduces bleeding.3,4
Proven Material
Unique biosynthetic scaffold designed to facilitate tissue generation.7
Strength and flexibility
The only staple line reinforcement material with trimethylene carbonate (TMC) to help maintain the strength with increased conformability.8
Infiltration to enable vascularization
Porous, 3D microstructure facilitates new vascularized soft tissue ingrowth.7
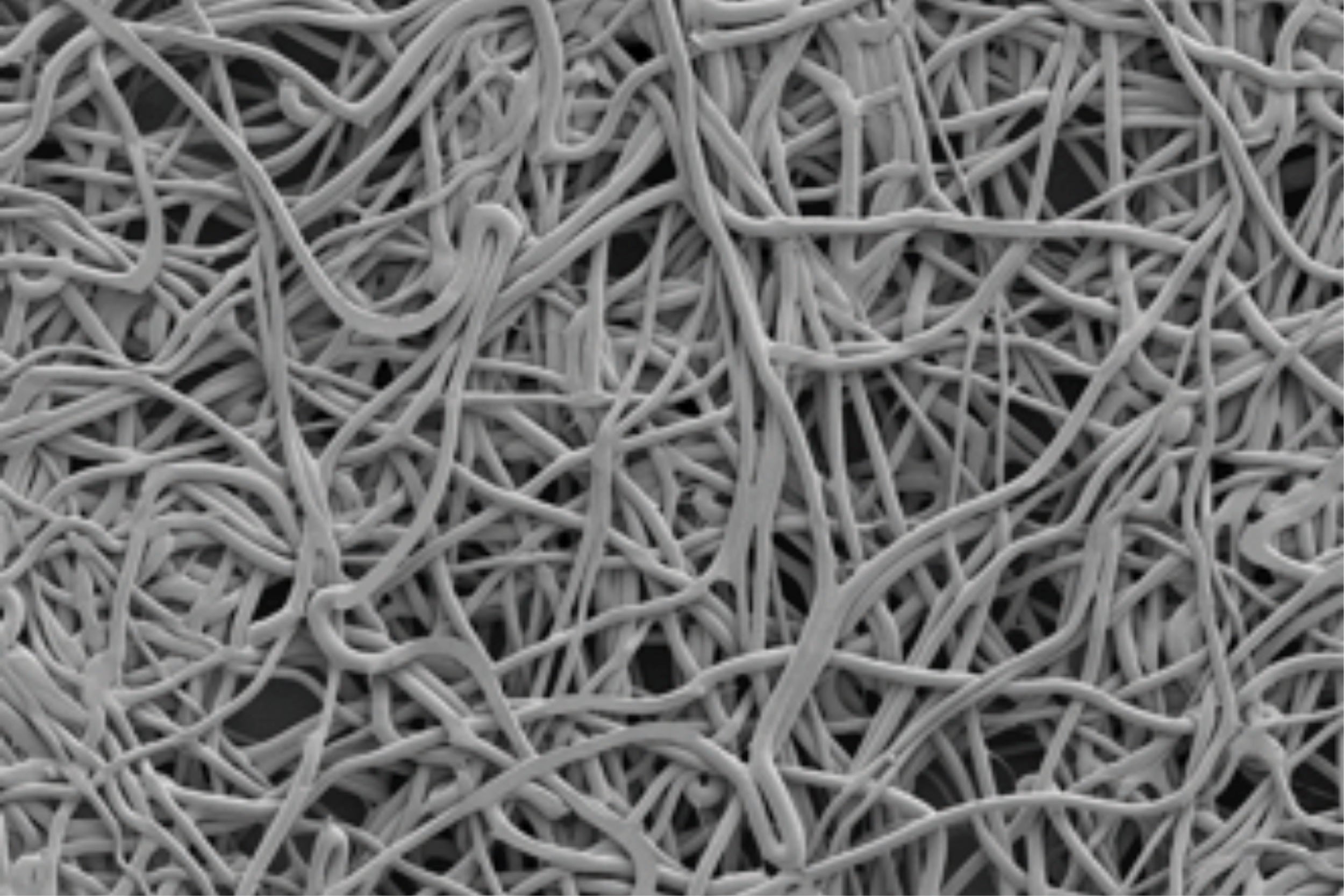
Scanning electron microscope image of GORE® SEAMGUARD® Bioabsorbable Staple Line Reinforcement Surface, 50X magnification.
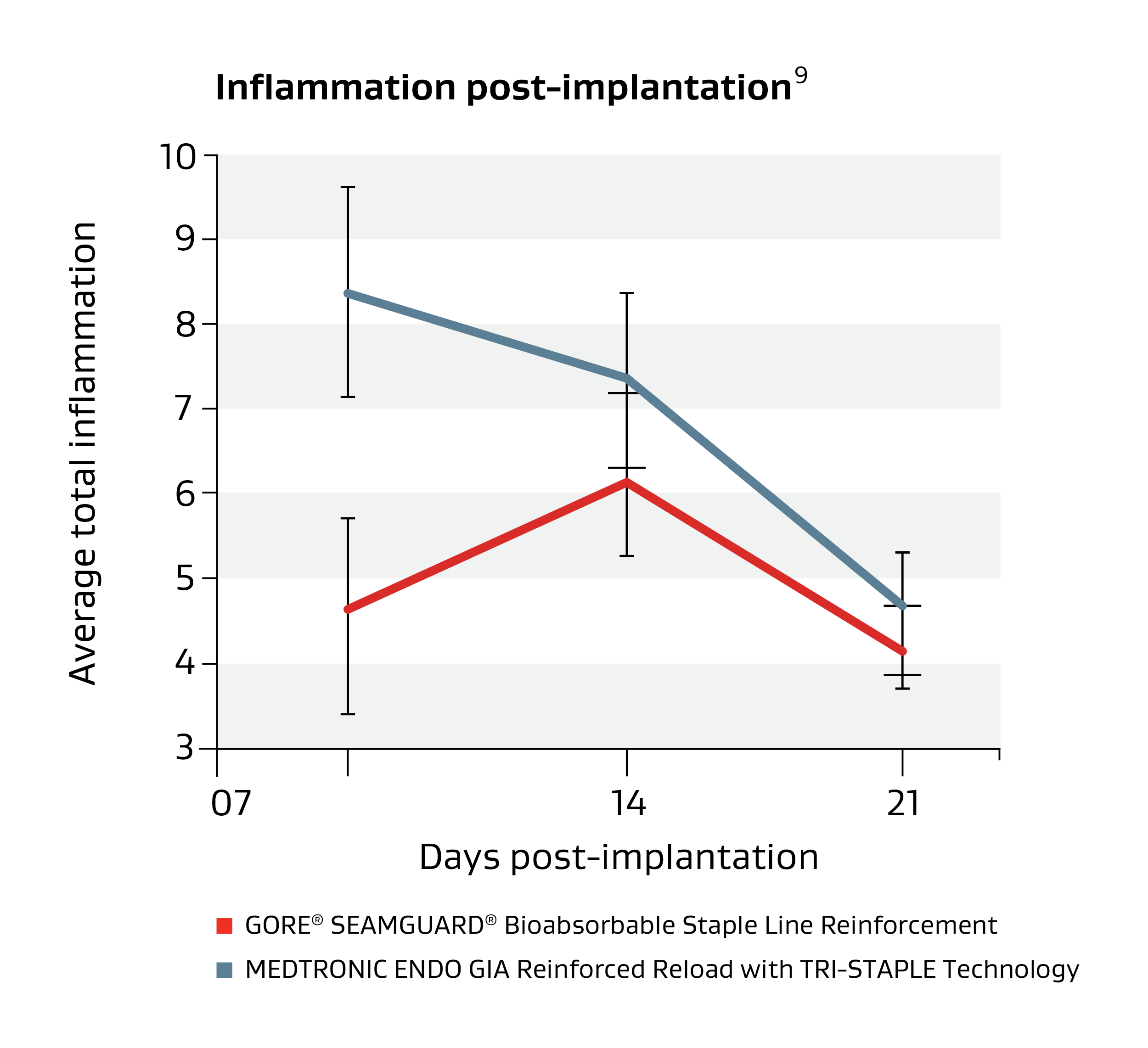
Minimal inflammation
Compared with similar materials it has shown minimal tissue inflammatory response.8-10
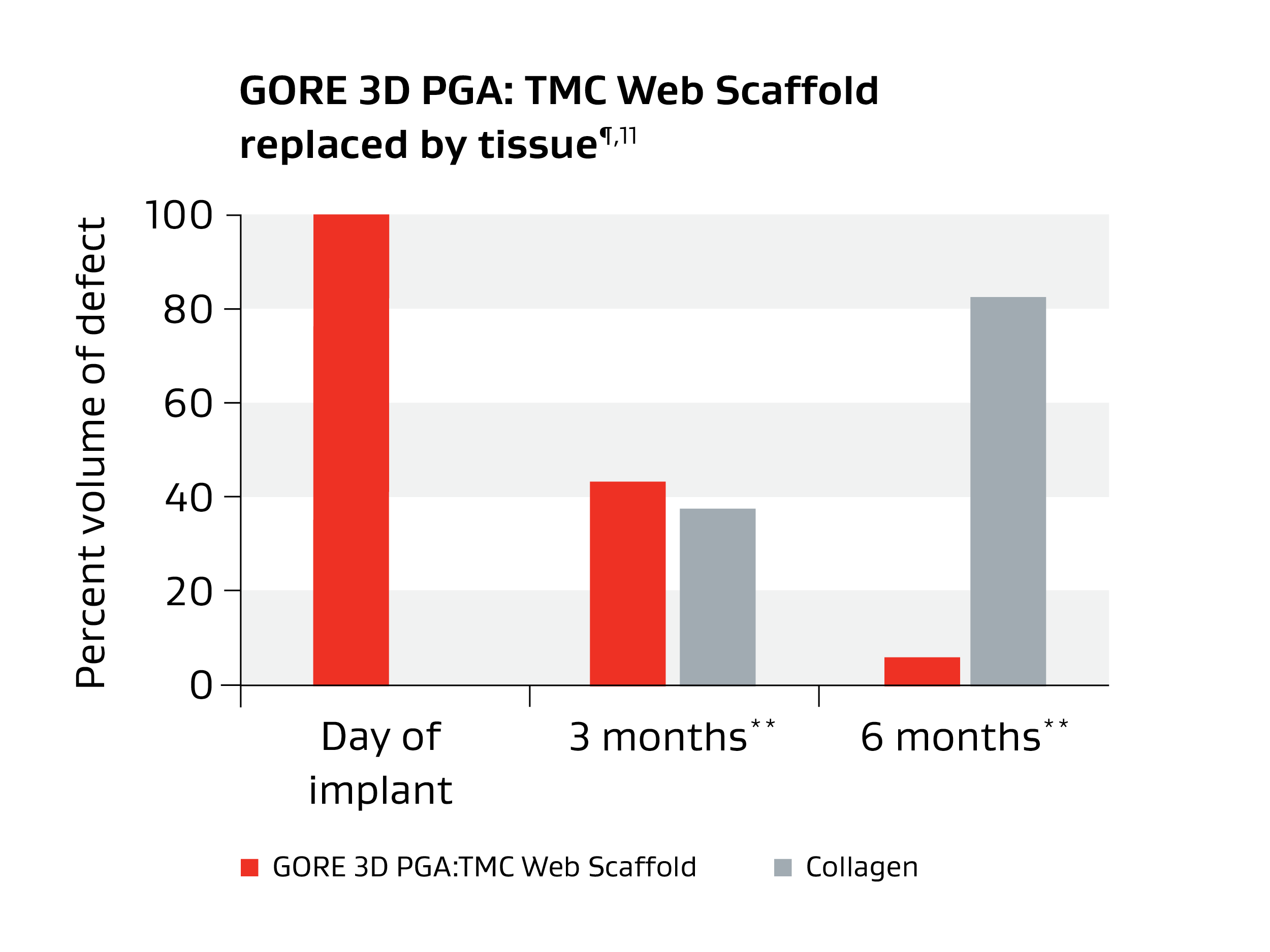
Unique bioabsorbable polymer
Serving as a scaffold, GORE® SEAMGUARD® Reinforcement successfully guides the regeneration of favorable Type 1 collagen while gradually absorbing within six to seven months.7,8
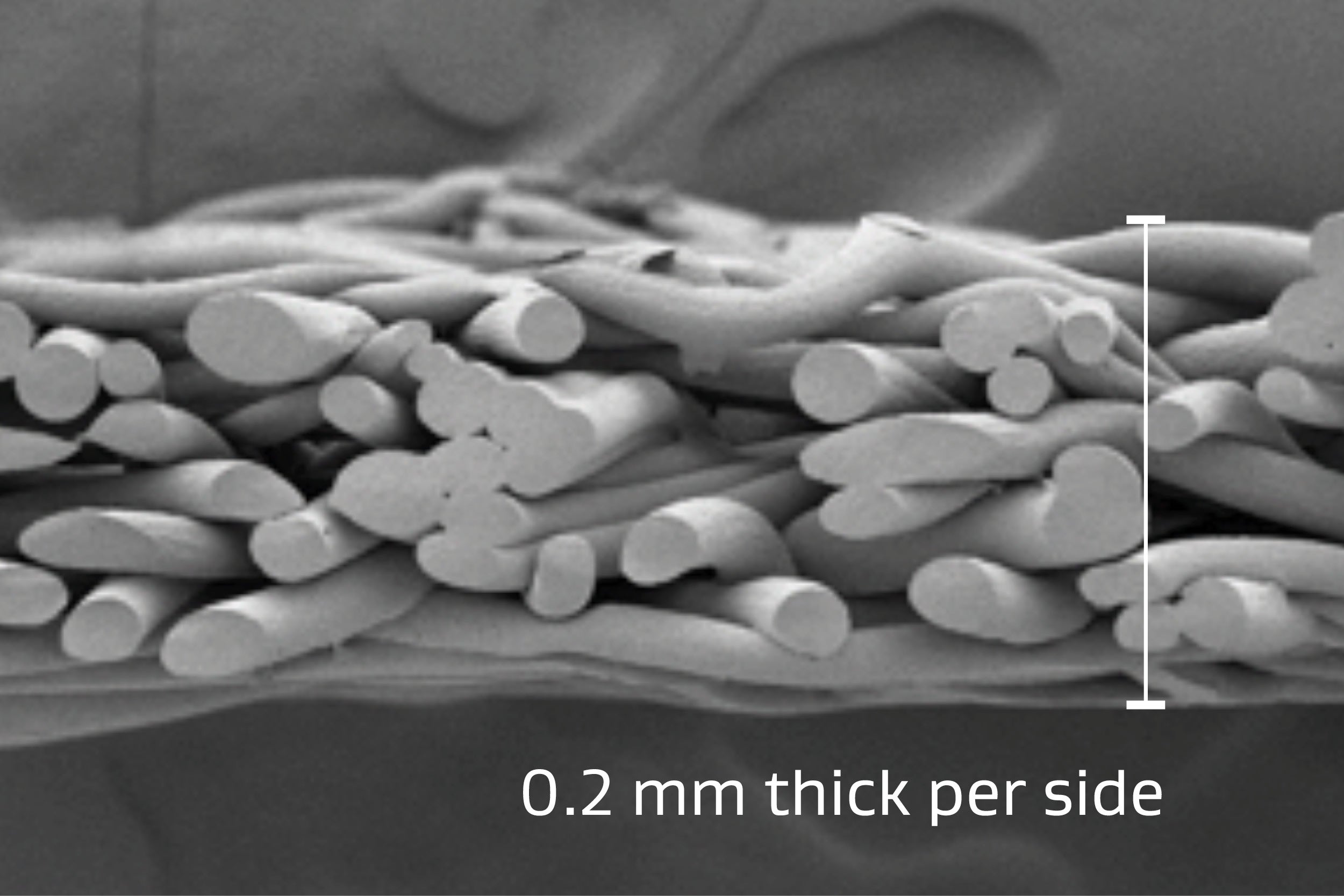
Material thickness
Thin, conformable and compressible
Consistent 0.4 mm average total thickness allowing for even pressure distribution over the tissue while minimizing impact on staple formation. The composition of GORE® SEAMGUARD® Reinforcement allows for the thickness to compress when more force is applied.12
Holds and protects
Strengthens staple lines with minimal inflammation.
Made with a synthetic buttressing material engineered to reduce perioperative leaks and bleeding.13 Compared with similar materials it has shown minimal tissue inflammatory response.8-10
* After 20 years,‡ over 5 million devices implanted† and more than 85 published and peer-reviewed clinical studies,§
† Data on file 2022; W. L. Gore & Associates, Inc; Flagstaff, AZ.
‡ First commercial marketing authorization, U.S. Food and Drug Administration in April 2003.
§
ll Robotic configurations are not available in Canada.
¶ Data on file 2015; W. L. Gore & Associates, Inc; Flagstaff, AZ.
** Cells and blood vessels make up remaining volume.
- W. L. Gore & Associates, Inc. Clinical Performance with Staple Line Reinforcement. Scientific Literature Analysis (n = 8142 patients). Flagstaff, AZ: W. L. Gore & Associates, Inc; 2023. [Literature summary]. 231029724-EN.
- Gagner M, Kemmeter P. Comparison of laparoscopic sleeve gastrectomy leak rates in five staple-line reinforcement options: a systematic review. Surgical Endoscopy 2020;34(1):396-407. https://rd.springer.com/article/10.1007/s00464-019-06782-2
- Miller KA, Pump A. Use of bioabsorbable staple reinforcement material in gastric bypass: a prospective randomized clinical trial. Surgery for Obesity & Related Diseases 2007;3(4):417-422.
- Nguyen NT. Longoria M, Welbourne S, Sabio A, Wilson SE. Glycolide copolymer staple-line reinforcement reduces staple site bleeding during laparoscopic gastric bypass. A prospective randomized trial. Archives of Surgery 2005;140(8):773-778
- Zambelli-Weiner A, Brooks E, Brolin R, Bour ES. Total charges for postoperative leak following laparoscopic sleeve gastrectomy. Presented at Obesity Week 2013: The American Society for Metabolic and Bariatric Surgery and the Obesity Society Joint Annual Scientific Meeting; November 11-16, 2013; Atlanta, GA. A-305-P.
- Gayrel X, Loureiro M, Skalli EM, Dutot C, Mercier G, Nocca D. Clinical and economic evaluation of absorbable staple line buttressing in sleeve gastrectomy in high-risk patients. Obesity Surgery 2016;26(8):1710-1716.
- Zemlyak AY, Colavita PD, Tsirline VB, et al. Absorbable glycolic acid/trimethylene carbonate synthetic mesh demonstrates superior in-growth and collagen deposition. Presented at the Abdominal Wall Reconstruction (AWR) Meeting; June 14-16, 2012; Washington, DC. Abstract 35.
- Katz AR, Mukherjee DP, Kaganov AL, Gordon S. A new synthetic monofilament absorbable suture made from polytrimethylene carbonate. Surgery, Gynecology & Obstetrics 1985;161(3):213-222.
- Khaitan L, Yoo J. Comparison Between Staple Line Reinforcement Materials: GORE® SEAMGUARD® Bioabsorbable Staple Line Reinforcement and MEDTRONIC ENDO GIA Reinforced Reload with TRI-STAPLE Technology. Flagstaff, AZ: W. L. Gore & Associates, Inc; 2017. [Case series]. AV1650-EN1.
- Crawford N. Assessment of Various Staple Line Reinforcement materials in a Porcine Model. Flagstaff, AZ: W. L. Gore & Associates, Inc; 2015. [Histopathology report]. Experiment 2284TL. T3 Protocol WG03P.
- Morales-Conde S, Flores M, Fernández V, Morales-Méndez S. Bioabsorbable vs polypropylene plug for the “Mesh and Plug” inguinal hernia repair. Poster presented at the 9th Annual Meeting of the American Hernia Society; February 9-12, 2005; San Diego, CA. AJ0049-EN2.
- Pertuit A. Bioabsorbable Seamguard Density study for Leak Testing. Flagstaff, AZ: W L. Gore & Associates. Inc; 2003. [Work plan] MD15039.
- McCrea C. GORE® SEAMGUARD® Reinforcement Product Family: Relationship between Design Inputs and “Engineered to reduce the incidence of perioperative leaks and bleeding” Marketing Statement. Flagstaff, AZ; 2015. [Work plan]. WP107241.
MEDTRONIC, ENDO GIA and TRI-STAPLE are trademarks of Medtronic, Inc.

Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. RXOnly
CONFIGURED FOR ENDOSCOPIC SURGICAL STAPLERS:
INDICATIONS FOR USE: GORE® SEAMGUARD® Bioabsorbable Staple Line Reinforcement is indicated for use in surgical procedures in which soft tissue transection or resection with staple line reinforcement is needed. GORE® SEAMGUARD® Bioabsorbable Staple Line Reinforcement can be used for reinforcement of staple lines during lung resection, bronchial, bariatric, colon, colorectal, gastric, mesentery, pancreas and small bowel procedures.
CONTRAINDICATIONS: Not for the patch reconstruction of cardiovascular defects such as cardiac, great vessel and peripheral vascular arteries or veins.
CONFIGURED FOR CIRCULAR SURGICAL STAPLERS:
INDICATIONS FOR USE: GORE® SEAMGUARD® Bioabsorbable Staple Line Reinforcement is indicated for use in surgical procedures in which a soft tissue anastomosis with staple line reinforcement is needed. GORE® SEAMGUARD® Bioabsorbable Staple Line Reinforcement can be used for reinforcement of staple lines during bariatric, colon, colorectal, gastric and small bowel procedures.
CONTRAINDICATIONS: Not for the reconstruction of cardiovascular defects such as cardiac, great vessel and peripheral vascular arteries or veins.
CONFIGURED FOR ROBOTIC ENDOSCOPIC SURGICAL STAPLERS:
INDICATIONS FOR USE: GORE® SEAMGUARD® Bioabsorbable Staple Line Reinforcement is indicated for use in surgical procedures in which soft tissue transection or resection with staple line reinforcement is needed. GORE® SEAMGUARD® Bioabsorbable Staple Line Reinforcement can be used for reinforcement of staple lines during lung resection, liver resection, bronchial, bariatric, colon, colorectal, esophagus, gastric, mesentery, pancreas, small bowel and spleen procedures.
GORE® SEAMGUARD® Bioabsorbable Staple Line Reinforcement is also intended to be used for reinforcement of suture-lines and staple-lines (i.e., occlusion of the left atrial appendage during open chest procedures) during cardiac surgery.
CONTRAINDICATIONS: Not for the patch reconstruction of cardiovascular defects such as cardiac, great vessel and peripheral vascular arteries or veins.
GORE® SEAMGUARD® Bioabsorbable Staple Line Reinforcement Configured for Robotic Endoscopic Surgical Staplers is not authorized for use in Canada.