GORE® SEAMGUARD® Bioabsorbable Staple Line Reinforcement Data Review: Bleeding
Protects against bleeding
Randomized prospective data has shown that the GORE® SEAMGUARD® Reinforcement significantly reduces bleeding.1,2
The GORE® SEAMGUARD® Reinforcement provides a matrix that blood can infiltrate. Platelets within the patient's blood stick together and create a clot to decrease bleeding.
Reduces overall bleeding complications
Randomized prospective clinical data
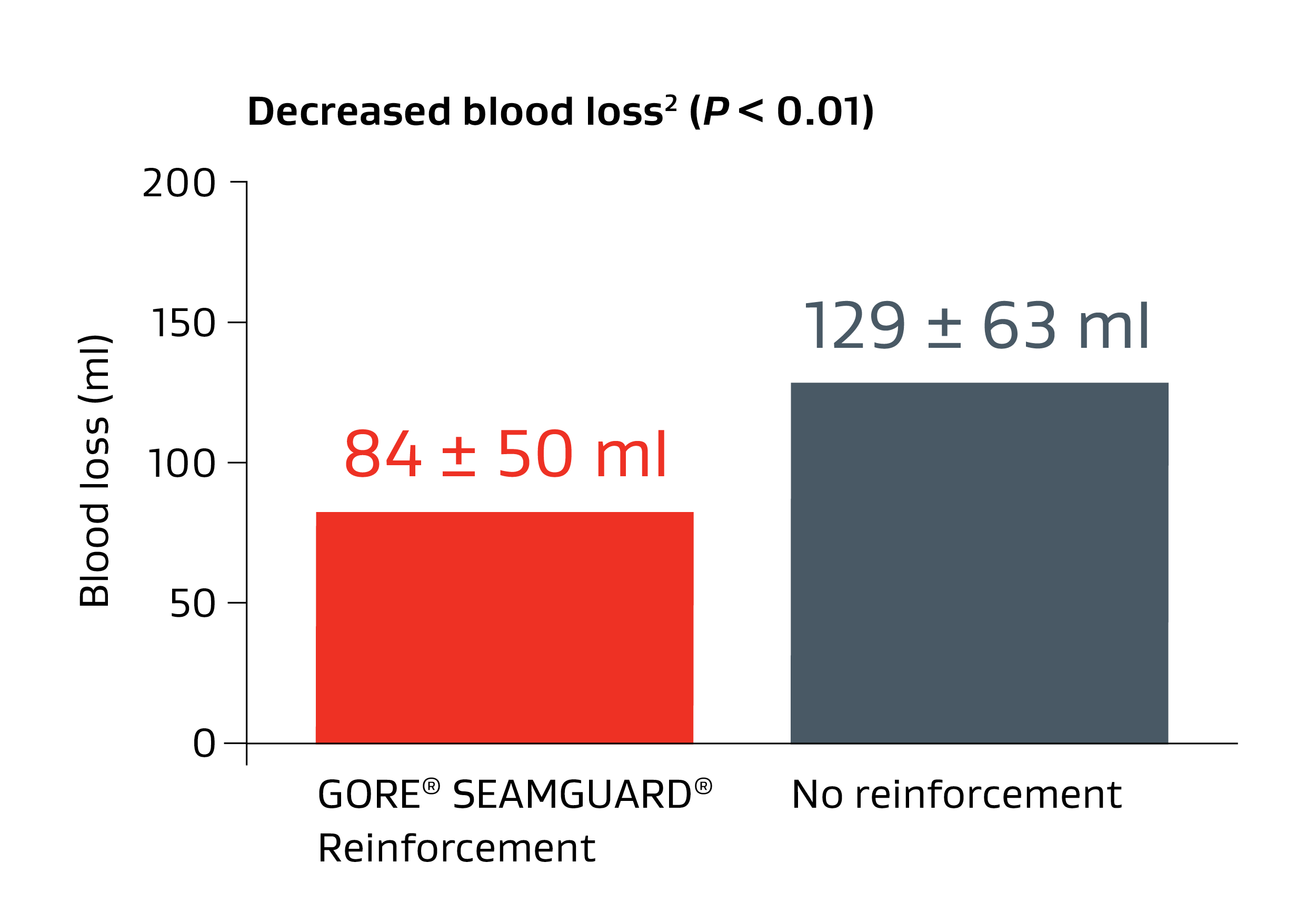
No reinforcement = 54% more bleeding on average 2
- Miller KA, Pump A. Use of bioabsorbable staple reinforcement material in gastric bypass: a prospective randomized clinical trial. Surgery for Obesity & Related Diseases 2007;3(4):417-422.
- Nguyen NT, Longoria M, Welbourne S, Sabio A, Wilson SE. Glycolide copolymer staple-line reinforcement reduces staple site bleeding during laparoscopic gastric bypass. A prospective randomized trial. Archives of Surgery 2005;140(8):773-778.
- Rosenthal RJ. International Sleeve Gastrectomy Expert Panel Consensus Statement. Best practice guidelines based on experience of over 12,000 cases. Surgery for Obesity & Related Diseases 2012;8(1):8-19.

Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. RXOnly
CONFIGURED FOR ENDOSCOPIC SURGICAL STAPLERS:
INDICATIONS FOR USE: GORE® SEAMGUARD® Bioabsorbable Staple Line Reinforcement is indicated for use in surgical procedures in which soft tissue transection or resection with staple line reinforcement is needed. GORE® SEAMGUARD® Bioabsorbable Staple Line Reinforcement can be used for reinforcement of staple lines during lung resection, bronchial, bariatric, colon, colorectal, gastric, mesentery, pancreas and small bowel procedures.
CONTRAINDICATIONS: Not for the patch reconstruction of cardiovascular defects such as cardiac, great vessel and peripheral vascular arteries or veins.
CONFIGURED FOR CIRCULAR SURGICAL STAPLERS:
INDICATIONS FOR USE: GORE® SEAMGUARD® Bioabsorbable Staple Line Reinforcement is indicated for use in surgical procedures in which a soft tissue anastomosis with staple line reinforcement is needed. GORE® SEAMGUARD® Bioabsorbable Staple Line Reinforcement can be used for reinforcement of staple lines during bariatric, colon, colorectal, gastric and small bowel procedures.
CONTRAINDICATIONS: Not for the reconstruction of cardiovascular defects such as cardiac, great vessel and peripheral vascular arteries or veins.
CONFIGURED FOR ROBOTIC ENDOSCOPIC SURGICAL STAPLERS:
INDICATIONS FOR USE: GORE® SEAMGUARD® Bioabsorbable Staple Line Reinforcement is indicated for use in surgical procedures in which soft tissue transection or resection with staple line reinforcement is needed. GORE® SEAMGUARD® Bioabsorbable Staple Line Reinforcement can be used for reinforcement of staple lines during lung resection, liver resection, bronchial, bariatric, colon, colorectal, esophagus, gastric, mesentery, pancreas, small bowel and spleen procedures.
GORE® SEAMGUARD® Bioabsorbable Staple Line Reinforcement is also intended to be used for reinforcement of suture-lines and staple-lines (i.e., occlusion of the left atrial appendage during open chest procedures) during cardiac surgery.
CONTRAINDICATIONS: Not for the patch reconstruction of cardiovascular defects such as cardiac, great vessel and peripheral vascular arteries or veins.
GORE® SEAMGUARD® Bioabsorbable Staple Line Reinforcement Configured for Robotic Endoscopic Surgical Staplers is not authorized for use in Canada.