
Measurable value for lower cost of care
GORE® PROPATEN® Vascular Graft for lower extremity revascularization is specifically designed to reduce the risk of acute graft thrombotic failure. It has a decade of strong performance with improving outcomes and reducing interventions, helping to deliver both proven clinical and economic value for patients and hospitals.
Proven Patency
By substantially reducing acute graft thrombosis within hours after implantation, the CBAS Heparin Surface on the GORE® PROPATEN® Vascular Graft provides clinical benefits that standard ePTFE grafts do not.1
Fewer occlusions
50% reduction in risk of graft occlusion compared to standard ePTFE in critical limb ischemia (CLI) patients.2
Improved patient outcomes
Higher primary and secondary patency, and higher limb salvage for below-knee bypass compared to standard ePTFE from 1–3 years.3
GORE® PROPATEN® Vascular Graft provides measurable value for lower cost of care
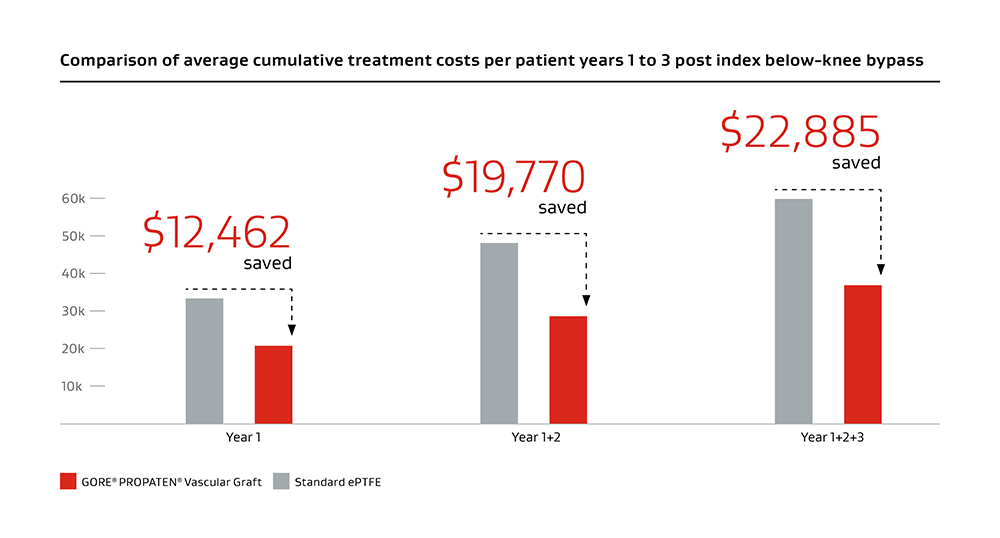
Includes data for graft purchase cost, graft reinterventions,* redo bypasses,† amputation procedures, amputation rehabilitation and care.
38%‡ providers
Decrease in average cost
The Future of Value Analysis
A Handbook for Health Care Professionals
Read perspectives from value analysis professionals who share their thoughts regarding the importance of effective collaboration, paradigm shifts with determining value, and the critical focus on the future of healthcare.

* Procedures for restoring flow in stenosed or occluded graft.
† Replacing the graft with new graft.
‡ Based on the 3-year published clinical performance and economic model.
- Biran R, Pond D. Heparin coatings for improving blood compatibility of medical devices. Advanced Drug Delivery Reviews 2017;112:12-23. https://www.sciencedirect.com/science/article/pii/S0169409X16303210
- Lindholt JS, Gottschalksen B, Johannesen N, et al. The Scandinavian PROPATEN® Trial – 1-year patency of PTFE vascular prostheses with heparin-bonded luminal surfaces compared to ordinary pure PTFE vascular prostheses – a randomized clinical controlled multi-centre trial. European Journal of Vascular & Endovascular Surgery 2011;41(5):668-673.
- GORE® PROPATEN® Vascular Graft. W. L. Gore & Associates Web site. https://www.goremedical.com/propaten/references. Accessed May 15, 2019.

Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. RXOnly
INDICATIONS FOR USE IN THE U.S.: GORE® PROPATEN® Vascular Grafts are intended for use as vascular prostheses for replacement or bypass of diseased vessels in patients suffering occlusive or aneurysmal diseases, in trauma patients requiring vascular replacement, for dialysis access, or for other vascular procedures.
CONTRAINDICATIONS: A. DO NOT use the GORE® PROPATEN® Vascular Graft in patients with known hypersensitivity to heparin, including those patients who have had a previous incidence of Heparin-Induced Thrombocytopenia (HIT) type II. B. DO NOT use any configuration of GORE® PROPATEN® Vascular Grafts with Removable Rings, Non-Removable Rings or Integrated Rings for coronary artery bypass or cerebral reconstruction procedures. C. DO NOT use GORE® PROPATEN® Vascular Grafts as a patch. If cut and used as a patch, GORE® PROPATEN® Vascular Grafts may lack adequate transverse strength.
