Catalogue numbers for GORE® DRYSEAL Flex Introducer Sheath
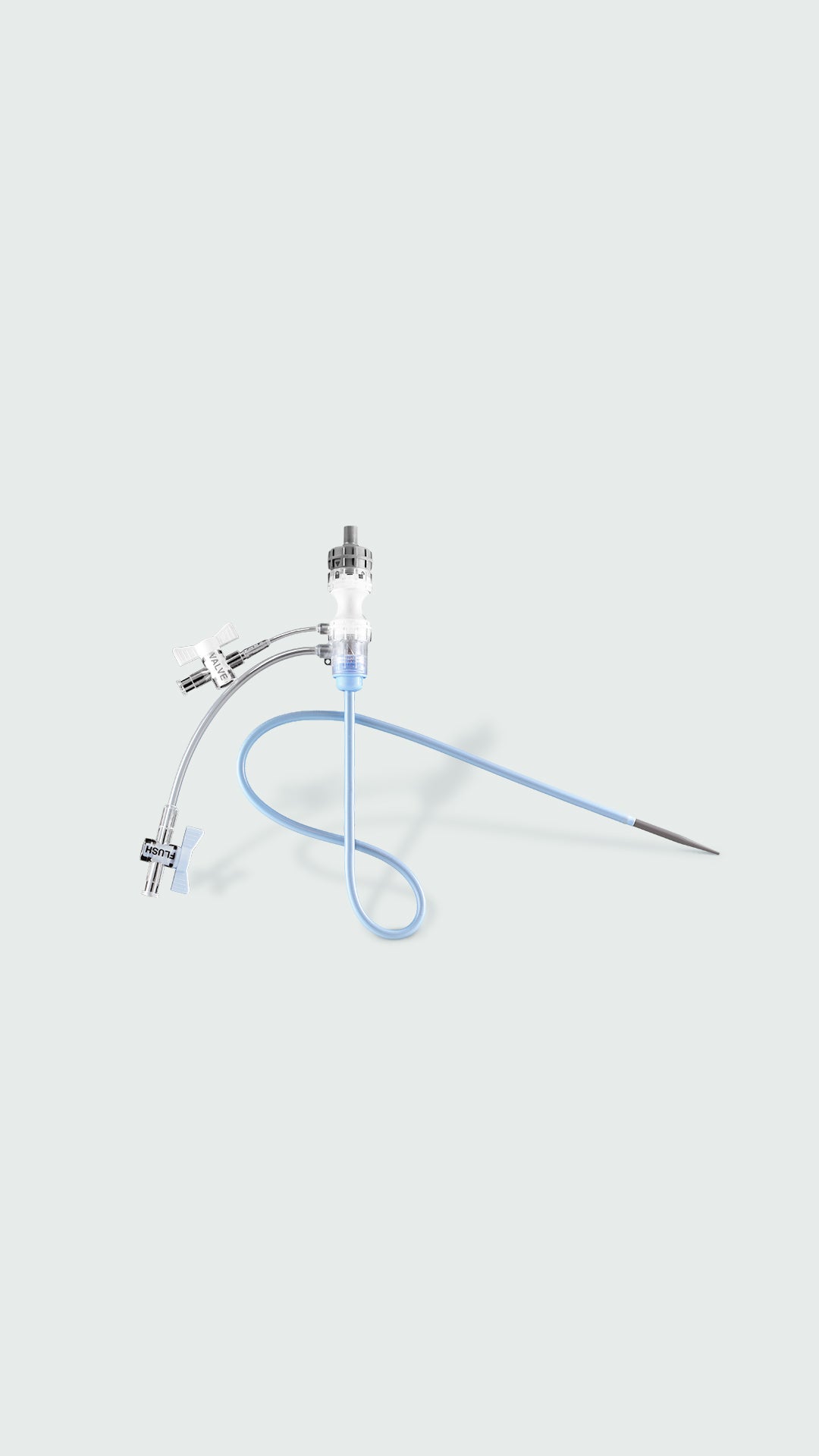
GORE® DRYSEAL Flex Introducer Sheath | ||||
|---|---|---|---|---|
| Catalogue Number* | Sheath Size (Fr) | Minimum Sheath ID (mm) | Nominal Body OD (mm) | Sheath Working Length (cm) |
| GDSF1033 | 10 | 3.3 | 4.0 | 33 |
| GDSF1045 | 10 | 3.3 | 4.0 | 45 |
| GDSF1065 | 10 | 3.3 | 4.0 | 65 |
| GDSF1233 | 12 | 4.0 | 4.7 | 33 |
| GDSF1245 | 12 | 4.0 | 4.7 | 45 |
| GDSF1265 | 12 | 4.0 | 4.7 | 65 |
| GDSF1433 | 14 | 4.7 | 5.3 | 33 |
| GDSF1465 | 14 | 4.7 | 5.3 | 65 |
| GDSF1533 | 15 | 5.0 | 5.6 | 33 |
| GDSF1633 | 16 | 5.3 | 6.1 | 33 |
| GDSF1665 | 16 | 5.3 | 6.1 | 65 |
| GDSF1833 | 18 | 6.0 | 6.7 | 33 |
| GDSF1865 | 18 | 6.0 | 6.7 | 65 |
| GDSF2033 | 20 | 6.7 | 7.5 | 33 |
| GDSF2065 | 20 | 6.7 | 7.5 | 65 |
| GDSF2233 | 22 | 7.3 | 8.2 | 33 |
| GDSF2265 | 22 | 7.3 | 8.2 | 65 |
| GDSF2433 | 24 | 8.0 | 8.8 | 33 |
| GDSF2465 | 24 | 8.0 | 8.8 | 65 |
| GDSF2633 | 26 | 8.7 | 9.5 | 33 |
| GDSF2665 | 26 | 8.7 | 9.5 | 65 |
*GDSF catalogue numbers are not available in all regions; use DSF catalogue numbers instead where appropriate.
Sizing, availability, and pricing varies by country.
Please check with your Gore representative for availability.

Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. RXOnly
INDICATIONS FOR USE IN THE U.S.: The GORE® DRYSEAL Flex Introducer Sheath is intended to be inserted in the vasculature to provide a conduit for the insertion of endovascular devices while minimizing blood loss associated with such insertions.
CONTRAINDICATIONS: There are no known contraindications for this device.