Non-healing ulcer with superficial femoral artery (SFA) disease
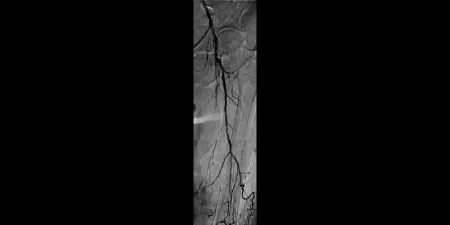
Challenge
- 72-year-old male with non-healing left foot ulceration (Rutherford 5) and leg edema
- Relevant patient history:
- Diabetes with peripheral neuropathy
- Congestive heart failure
- Prior interventions:
- February 2009 - Left femoral to below-knee bypass with right saphenous vein (claudication)
- September 2010 - Vein bypass thrombosed, with unsuccessful attempt to treat the graft at outside institution. Developed non-healing ulcer on top of left foot. Calcified ankle-brachial indexes (ABIs), with toe pressure of 20 mmHg on left and 34 mmHg on right. No palpable pulses below left femoral level.
- October 2011 - Left SFA treatment
Image: Left SFA chronic total occlusion (CTO) with one-vessel runoff (not shown)
Images courtesy of Bruce Gray, MD. Used with permission.
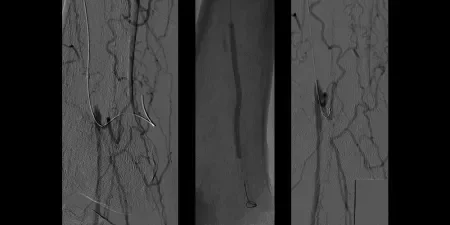
Procedure
- Subintimal traversal without re-entry, followed by percutaneous transluminal angioplasty (PTA) and then re-entry using a CORDIS® OUTBACK® Device
- Deployed two 6 mm x 15 cm GORE® VIABAHN® Devices
Images courtesy of Bruce Gray, MD. Used with permission.
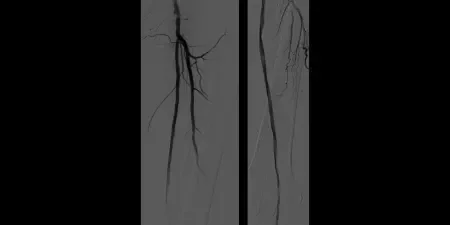
Result
- Maintained one-vessel runoff
- The patient went home after three hours with palpable dorsalis pedis pulse, ABI of 1.0
- Long-term anticoagulation / antiplatelet regimen: Warfarin and ASPIRIN (acetylsalicylic acid) Surveillance with duplex ultrasound within one month then at six months
- No recurrence of ulceration or need for other interventions and no amputations
- The patient died 11 months later (in September 2012) of respiratory failure associated with his congestive heart failure
Image: Post-placement of two 6 mm x 15 cm GORE® VIABAHN® devices. Maintained one-vessel runoff (not shown)
Images courtesy of Bruce Gray, MD. Used with permission.
Case takeaways
- Successful recanalization of SFA CTO in critical limb ischemia (CLI) patient
- Good inline flow through the SFA with preservation of tibial runoff
- Healing of ulcer and limb preservation in CLI patient
- Consider duplex ultrasound at frequent intervals through the first year, especially when runoff is limited
CORDIS® and OUTBACK® are trademarks of Cordis Corp.
The outcomes and observations reported are based on individual case experience and the patients treated. The steps described here may not be complete, and are not intended to be a replacement for the Instructions for Use or the education, training and professional judgment of Healthcare Providers. Healthcare Providers remain solely responsible for making decisions about patient care and the use of medical technologies.
Refer to Instructions for Use at eifu.goremedical.com for complete description of all indications, warnings, precautions and contraindications for the markets where this product is available.