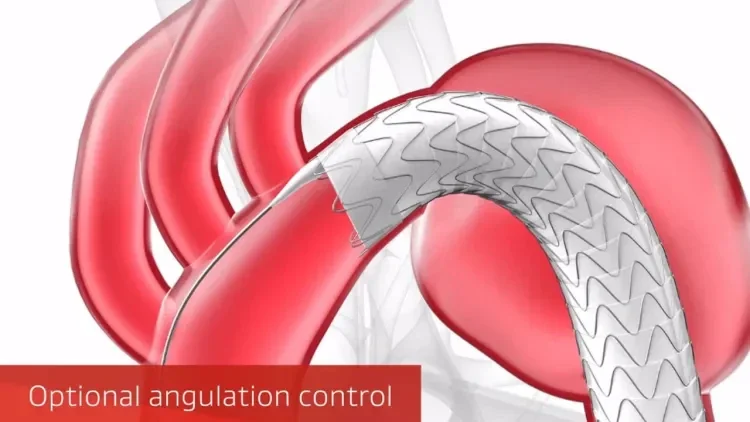
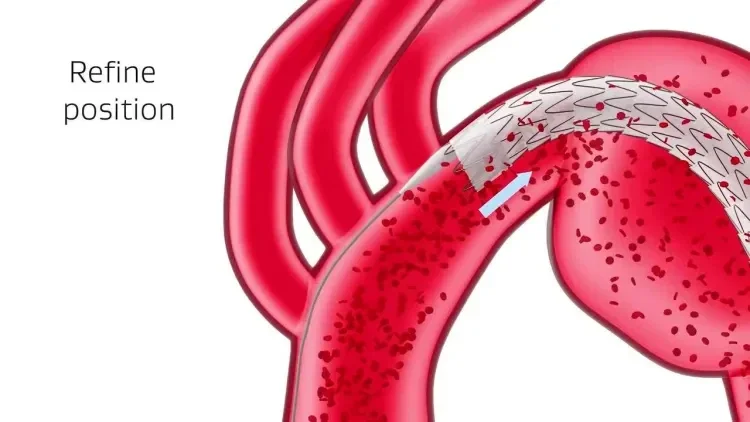
GORE® TAG® Conformable Thoracic Stent Graft with ACTIVE CONTROL System
Combining a durable, proven stent graft with a delivery system that offers controlled, staged deployment for the endovascular repair of aneurysms, transections and Type B dissections of the thoracic aorta.
The new delivery system allows accurate device positioning and location refinement during the intermediate stage before full deployment, and continuous blood flow throughout the procedure.
Oversizing matters


Featured Resources

IDE 5 year data brochure
Newly published 5 year data for aneurysm, dissection and transection clinical trials
PDF, 17.58 MB
* Migrations are reported as those requiring reintervention.
1. W. L. Gore & Associates. Evaluation of the GORE Conformable TAG® Thoracic Endoprosthesis for Treatment of Acute Complicated Type B Aortic Dissection. NLM Identifier: NCT00908388. Published May 25, 2009. Updated October 27, 2017. Accessed June 7, 2022. Available from: https://clinicaltrials.gov/ct2/show/NCT00908388
2. W. L. Gore & Associates. Evaluation of the GORE Conformable TAG® for Treatment of Traumatic Transection. NLM Identifier: NCT00917852. Published June 10, 2009. Updated August 25, 2017. Accessed June 7, 2022. https://clinicaltrials.gov/ct2/show/NCT00917852
3. W. L. Gore & Associates. An Evaluation of the GORE Conformable TAG® Thoracic Endoprosthesis for the Primary Treatment of Aneurysm of the Descending Thoracic Aortic. NLM Identifier: NCT00874250. Published April 2, 2009. Updated August 25, 2017. Accessed June 7, 2022. https://clinicaltrials.gov/ct2/show/NCT00874250

Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for markets where this product is available. RXOnly
231157293-EN