Trusted closure performance at 6 months*,1,2

| Characteristics of complex ASDs | |
|---|---|
| ASD Category | Anatomical Characteristics |
| Complex |
|
No retro-aortic rim requirements2
Gore ASSURED Clinical Study:
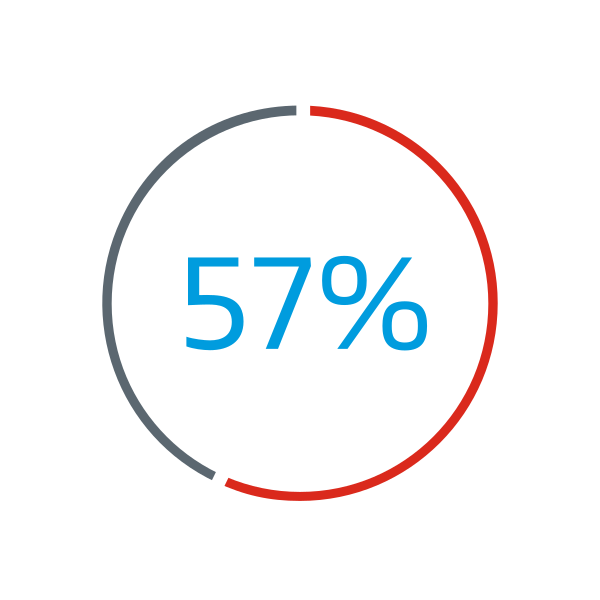
of patients enrolled in the Gore ASSURED Clinical Study were reported to have deficient retro-aortic rims (< 5 mm)1

effective closure at 6 months*,1,2
GORE® CARDIOFORM ASD Occluder
No warnings, potential adverse events or precautions related to cardiac erosions or use in patients with deficient retro-aortic rims.†,1,2
Download brochure for more information
Case examples
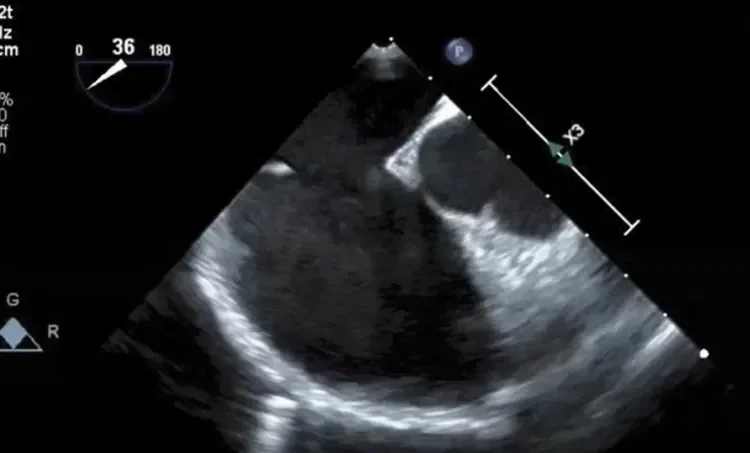
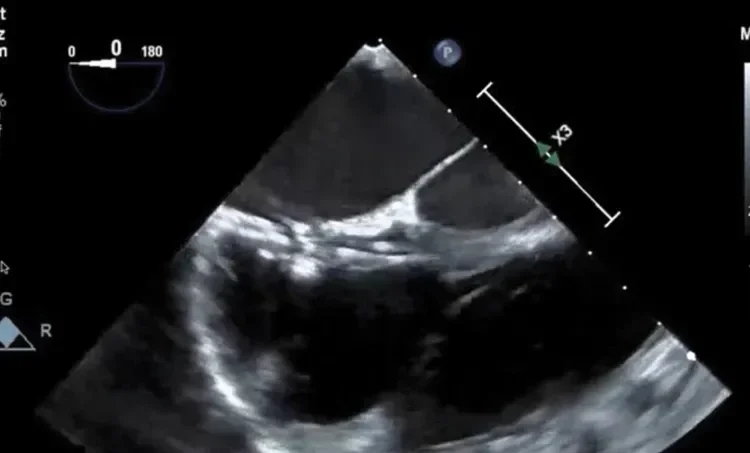
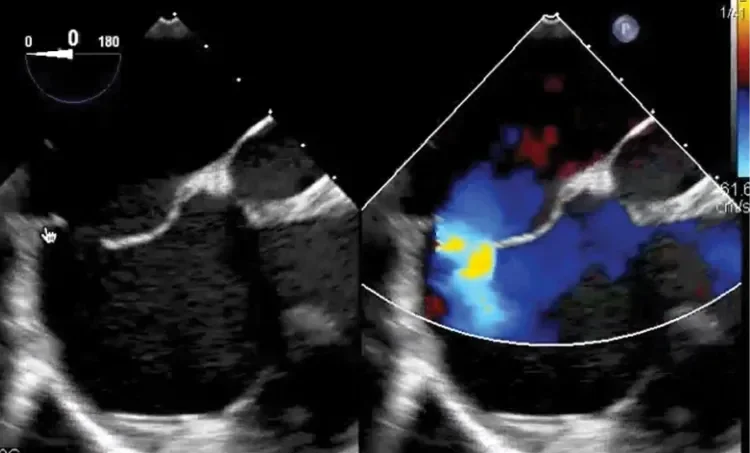
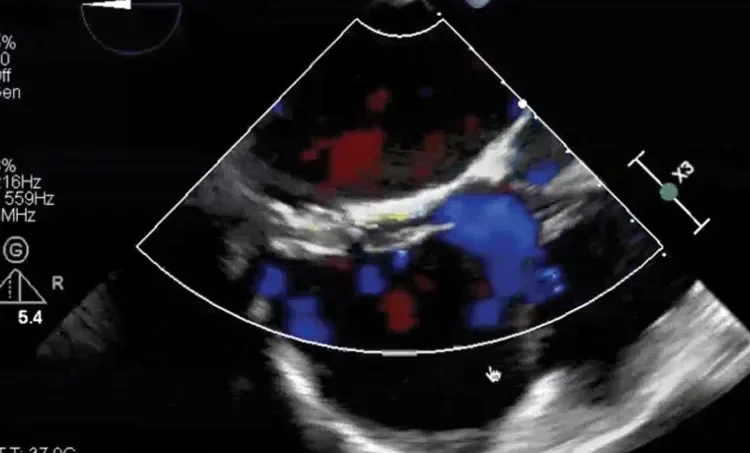
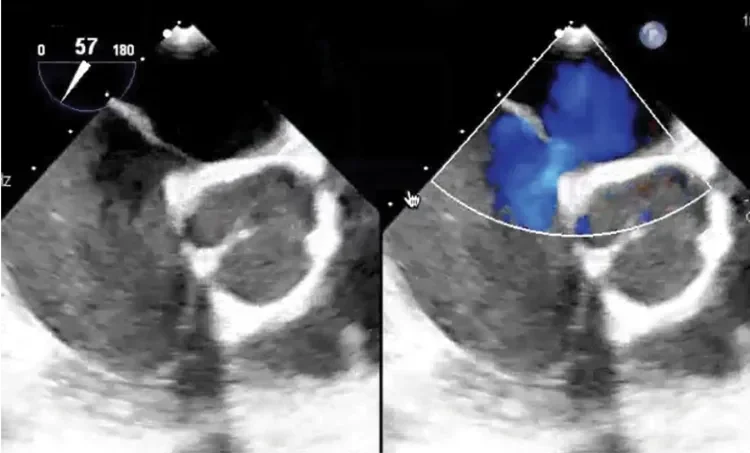
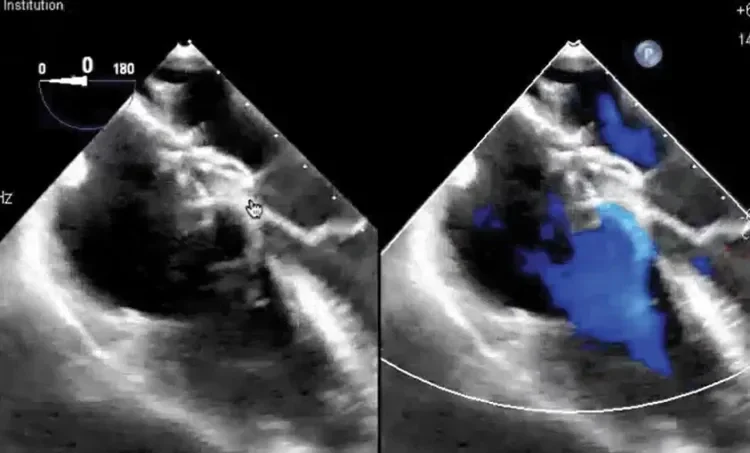
Deficient retro-aortic rim < 5 mm
Large ASD with deficient posterior-inferior rim
Multiple defects with atrial septal aneurysm (ASA)
See why we are a leader in safety
GORE® CARDIOFORM ASD Occluder Product Brochure
* Closure success defined as completely occluded or clinically insignificant shunt as determined by the Echo Core Lab at the 6-month evaluation among subjects with technical success.2
† Deficient retro-aortic rim was defined as a retro-aortic rim measuring less than or equal to 5 mm on any view on echocardiogram.
- Sommer RJ, Love BA, Paolillo JA, et al.; ASSURED Investigators. ASSURED clinical study: new GORE® CARDIOFORM ASD Occluder for transcatheter closure of atrial septal defect. Catheterization & Cardiovascular Interventions 2020;95(7):1285-1295.
- GORE® CARDIOFORM ASD Occluder [Instructions for Use]. Flagstaff, AZ.: W. L. Gore & Associates, Inc; 2024. MD200690.
- Hijazi ZM, Feldman T, Mustafa H, et al. Transcatheter Closure of ASDs and PFOs: A Comprehensive Assessment. 1st Edition. Cardiotext Publishing. 2010.
![]()
Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. RXOnly
24CR2023-EN01