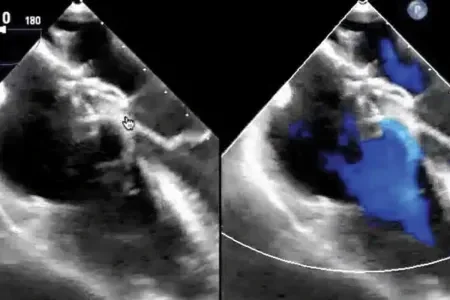
A leader in safety

GORE® CARDIOFORM ASD Occluder Product Brochure
* Reported incidence rate of device-related cardiac erosions for GORE® CARDIOFORM ASD Occluder calculated using data from CATSWeb® Product Surveillance Tracking System [PSTS] and Smartsolve.® Data on file. March 1, 2015 – July, 31,2024; W. L. Gore & Associates Inc.; Flagstaff, AZ.
- Sommer RJ, Love BA, Paolillo JA, et al.; ASSURED Investigators. ASSURED clinical study: new GORE® CARDIOFORM ASD Occluder for transcatheter closure of atrial septal defect. Catheterization & Cardiovascular Interventions 2020;95(7):1285-1295.
![]()
Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. RXOnly
CATSWeb is a trademark of AssurX, Inc.
SMARTSOLVE is a trademark of Ethos Technologies Inc.
24CR2024-EN01