
Trusted Outcomes
Delivering real-world evidence
Outcomes you can trust
The Global Registry for Endovascular Aortic Treatment (GREAT) is a comprehensive registry encompassing 28 aortic pathologies. With a 10-year patient follow-up, it provides trustworthy long-term results. The registry consists of real-world data from over 5,000 patients resulting in over 30 peer-reviewed publications in the past decade.


This, coupled with more than 25 years of clinical experience with aortic devices and over 20 years patient follow-up,1 offers an outstanding data pool. It supports health care providers with insights that can improve clinical decision making.
The major value of the GREAT Registry is having the opportunity to report on the very long-term follow-up, up to 10 years on more than 5,000 patients affected by thoracic and abdominal aortic disease.
Santi Trimarchi, M.D., Ph.D., Professor of Vascular Surgery,
University of Milan

This data includes procedural and clinical outcomes, covering 28 different pathologies along the aorta, as well as information on the devices used, patient medical history, demographics and disease state.
The ability to track such a large number of patients over an extended period of time is something that clearly sets GREAT apart from other registries. This sample will provide us with an opportunity to measure patient successes and outcomes that help us make continued improvements in aortic endovascular repair.
Dennis Gable M.D., FACS, DFSVS
Chief of Vascular and Endovascular Surgery
Baylor Scott & White Heart Hospital Plano
TCU Burnett School of Medicine
Professor of Surgery

The largest registry in the aortic field
Dennis R. Gable, M.D.
Ross Milner, M.D.
Device construction, materials and stent design all have significant impact on the overall outcome of aortic procedures. The GORE® EXCLUDER® AAA Endoprosthesis demonstrates this importance with its proven track record of 0.1% migration and fracture rates along with very low type I, III and IV endoleak rates.2
Ross Milner, M.D., Section of Vascular
Surgery and Endovascular Therapy;
Vice Chair, Peri-operative Services and Clinical Affairs,
University of Chicago Medicine

Long-term durability for TEVAR and EVAR
TEVAR
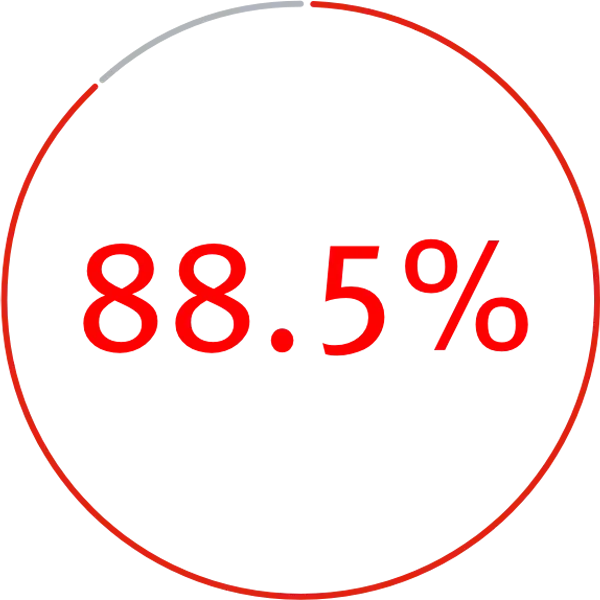
Freedom
from device-related reinterventions with the Conformable GORE® TAG® Thoracic Endoprosthesis* through 5-year follow-up
| TEVAR | |
|---|---|
| Procedural | |
| Patients ° | 778 |
| Procedural survival | 99.7% |
| Access complication | 2.8% |
| Through 5 years | |
| Migration | 0.3% |
| Fracture | 0% |
| Compression | 0% |
| Conversion to open repair | 1.8% |
| Aortic-related mortality | 3% |
EVAR

Freedom
from device-related reinterventions with GORE® EXCLUDER® AAA Endoprosthesis† through 5-year follow-up
| EVAR | |
|---|---|
| Through 5 years | |
| Patients ° | 3,216 |
| Migration | 0.1% |
| Type 1 A endoleak | 1.1% |
| Type III endoleak | 0.2% |
| Conversion to open repair | 0.9% |
| Aortic-related mortality | 1.2% |
| Limb occlusion | 0.7% |
Helping to make more informed treatment decisions
Our team is available to provide specific data upon request in an effort to aid physicians in making more informed treatment decisions for their patients.

* W. L. Gore & Associates. 'GREAT' Global Registry for Endovascular Aortic Treatment - Outcomes Evaluation. Bethesda, MD: National Library of Medicine; 2012. Available from: NLM Identifier: NCT01658787. Published August 7, 2012. Updated: September 29, 2021. Accessed June 7, 2022. https://clinicaltrials.gov/ct2/show/NCT01658787
† GREAT. n = 4,997. To calculate the overall event rates from procedure through end of study period, all subjects who could have had events, regardless of length of follow-up, were included. For outcome data, GREAT only collects site reported serious adverse events.
- Van Gool F, Houthoofd S, Mufty H, Bonne L, Fourneau I, Maleux G. Long-term outcome results after endovascular aortoiliac aneurysm repair with the bifurcated EXCLUDER Endoprosthesis. J Vasc Surg. 2022;75(6):1882-1889.
- Long C, Katsargyris A, Milner R, Verhoeven E. Five-year results for abdominal aortic aneurysm repair with the GORE® EXCLUDER® device: Insights from the Gore Global Registry for Endovascular Aortic Treatment (GREAT). Annals of Vascular Surgery 2024;106:247-254.
24AR2074-EN02

