
Addressing real-world challenges in AV Access
Longevity on dialysis depends on reliable vascular access1
PTA as a first-line treatment can be helpful, but with recoil and the challenges we see with these patients, you really need to come up with something more definitive.
- Nicolas Mouawad, M.D.
Percutaneous transluminal angioplasty (PTA) presents significant limitations when treating arteriovenous (AV) access lesions. In fact, with each successive PTA procedure, patency rates diminish.2,3
As PTA effectiveness diminishes, intervention frequency increases2,3
In 1 survey, 57% of hemodialysis patients cited "the time dialysis takes out of my life" as their biggest frustration.4 Where possible, minimizing the frequency of access-related procedures may help optimize the patient experience.
New peer perspectives
Hear fellow physicians share their perspectives on PTA and the potential to improve the dialysis experience for patients.
Breaking the stenosis cycle
In treatment of > 50% AVG stenosis, the GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface*,† had nearly 50% higher primary patency at 6 months than PTA alone.3
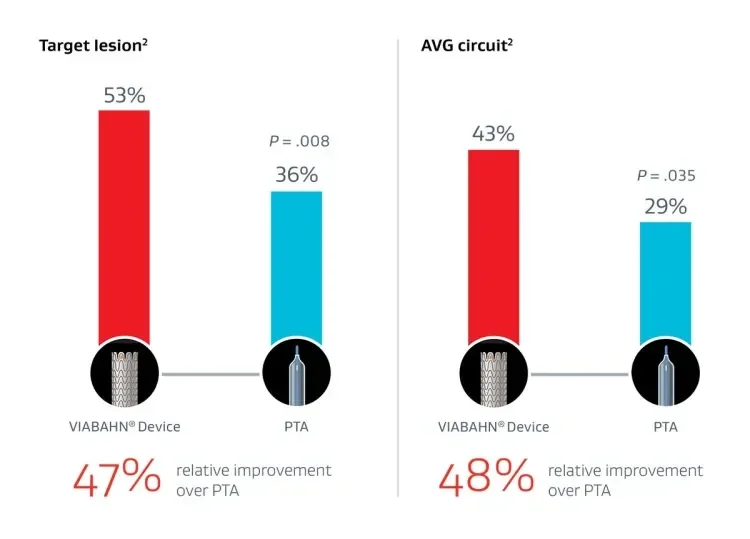
Changing course in stenosis with thrombosis
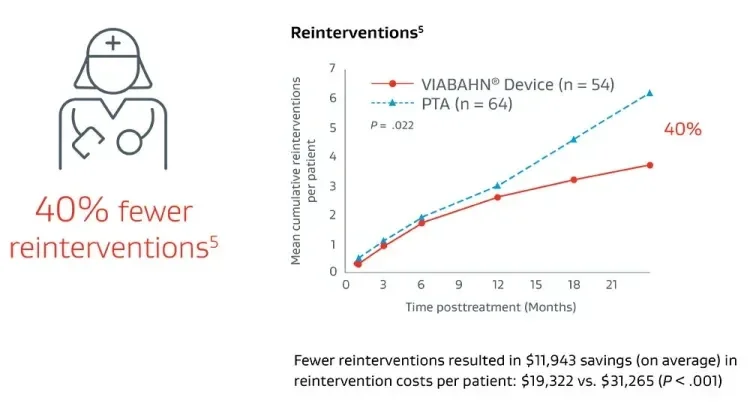
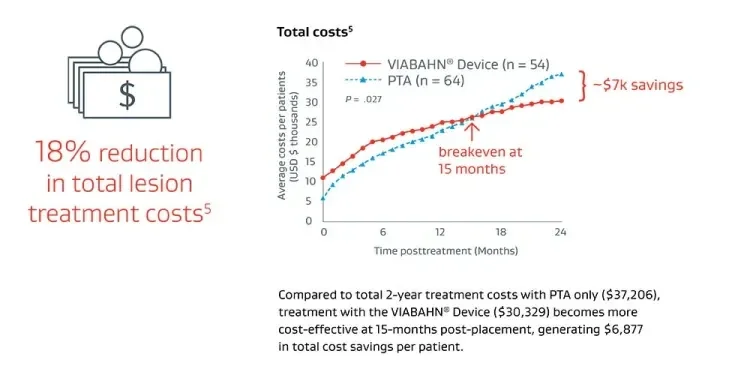
Thrombosed graft lesions treated with the VIABAHN® Device required fewer reinterventions and drove lower costs versus PTA at 2 years.5
Key KDOQI Recommendations:1
KDOQI recommends stent graft use in preference to angioplasty alone for recurrent clinically significant AVG stenosis and thrombotic AVG lesions.1

Stent grafts in preference to angioplasty alone for recurrent clinically significant stenotic and recurrent thrombotic AVG lesions.
Target goal of ≤ 3 interventions annually to maintain the access.

Avoid use of bare metal stents for the treatment of significant AVG and AVF stenotic lesions
* As used by Gore, Heparin Bioactive Surface refers to Gore’s proprietary CBAS® Heparin Surface.
† Also referred to as the GORE® VIABAHN® Endoprosthesis with PROPATEN Bioactive Surface in some regions.
- Lok CE, Huber TS, Lee T, et al; KDOQI Vascular Access Guideline Work Group. KDOQI Clinical Practice Guideline for Vascular Access: 2019 update. American Journal of Kidney Diseases 2020;75(4) Supplement 2:S1-S164. https://www.ajkd.org/article/S0272-6386(19)31137-0/fulltext
- Vesely T, DaVanzo W, Behrend T, Dwyer A, Aruny J. Balloon angioplasty versus Viabahn stent graft for treatment of failing or thrombosed prosthetic hemodialysis grafts. Journal of Vascular Surgery 2016;64(5):1400-1410.e1. http://www.sciencedirect.com/science/article/pii/S0741521416301756
- Kanterman RY, Vesely TM, Pilgram TK, Guy BW, Windus DW, Picus D. Dialysis access grafts: anatomic location of venous stenosis and results of angioplasty. Radiology 1995;195(1):135-139.
- Dialysis Access TAC, Physician and Patients Quantitative Research Report, April 2019. n=104
- Mohr BA, Sheen AL, Roy-Chaudhury P, Schultz SR, Aruny JE; REVISE Investigators. Clinical and economic benefits of stent grafts in dysfunctional and thrombosed hemodialysis access graft circuits in the REVISE Randomized Trial. Journal of Vascular & Interventional Radiology 2019;30(2):203-211.e4.https://www.jvir.org/article/S1051-0443(18)31772-X/fulltext

INDICATIONS FOR USE IN THE U.S.: The GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface is indicated for improving blood flow in patients with symptomatic peripheral arterial disease in superficial femoral artery de novo and restenotic lesions up to 270 mm in length with reference vessel diameters ranging from 4.0 – 7.5 mm. The GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface is indicated for improving blood flow in patients with symptomatic peripheral arterial disease in superficial femoral artery in-stent restenotic lesions up to 270 mm in length with reference vessel diameters ranging from 4.0 – 6.5 mm. The GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface is indicated for improving blood flow in patients with symptomatic peripheral arterial disease in iliac artery lesions up to 80 mm in length with reference vessel diameters ranging from 4.0 – 12 mm. The GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface is also indicated for the treatment of stenosis or thrombotic occlusion at the venous anastomosis of synthetic arteriovenous (AV) access grafts. CONTRAINDICATIONS: The GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface is contraindicated for non-compliant lesions where full expansion of an angioplasty balloon catheter was not achieved during pre-dilatation, or where lesions cannot be dilated sufficiently to allow passage of the delivery system. Do not use the GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface in patients with known hypersensitivity to heparin, including those patients who have had a previous incident of Heparin-Induced Thrombocytopenia (HIT) type II. Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. RXOnly
231145402-EN