GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis
The lower profile VBX Stent Graft is available only in the United States.
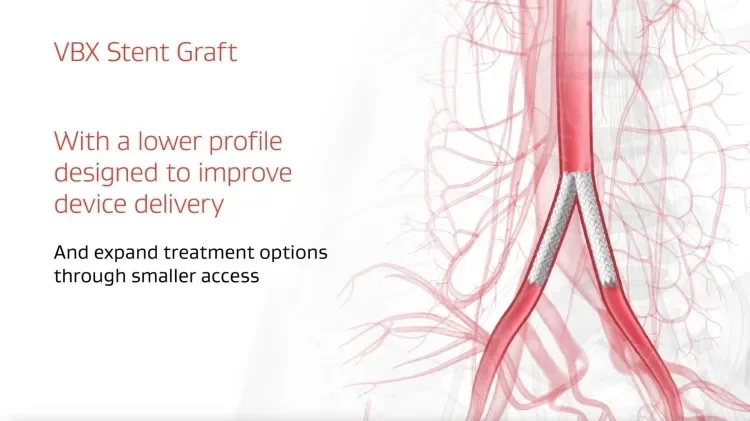
Designed to expand to every demand, the GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis (VBX Stent Graft) offers proven procedural success and long-term outcomes through flexibility, strength and accuracy to treat aortoiliac occlusive disease.
Improvements to the device delivery system have enabled a 1 Fr profile reduction on the majority of sizes, now offering the most 6 Fr compatible configurations among balloon expandable stent grafts.1-3


Darren Schneider, M.D., Andrew Holden, M.D., Bill Gray, M.D., and Venita Chandra, M.D. discuss benefits of the reduced profile VBX Stent Graft.
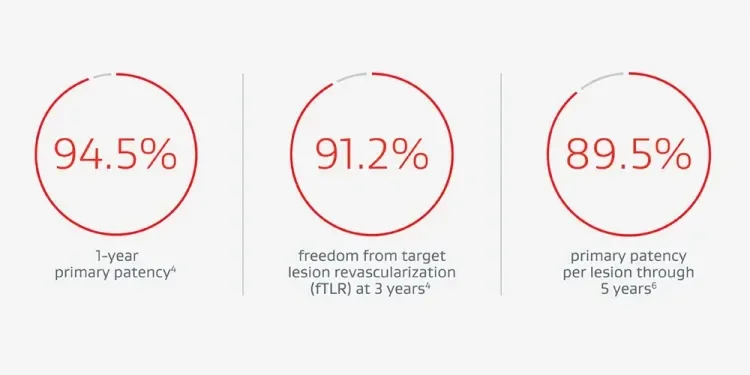
Trusted procedural and clinical performance

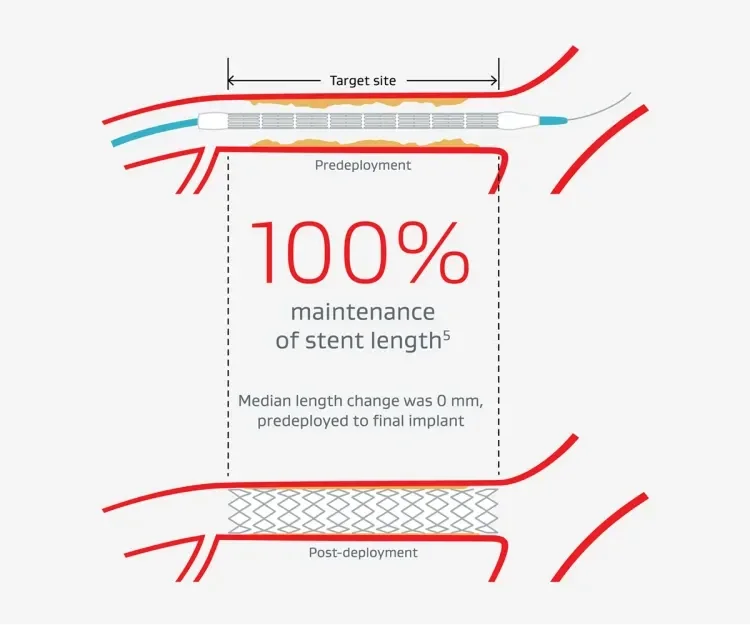
- 100% delivery to target lesion with no device dislodgement5
- 100% stent retention5
- 100% deployment at the target site5
- 234 devices delivered
- 50% bilateral treatment
- 42% kissing stents
- 32% TASC II C & D including occlusions
- 18% contralateral deliveries
- Predilatation not required

* Across indication inclusivity and configuration breadth/capability of balloon expandable covered stents.
- GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis [Instructions for Use]. Flagstaff, AZ: W. L. Gore & Associates, Inc; 2023. MD169334.
- LIFESTREAM® Balloon Expandable Vascular Covered Stent [Instructions for Use]. Tempe, AZ: Bard Peripheral Vascular, Inc; 2019. BAW1345700 Rev. 5 06/19.
- iCast covered stent system [Instructions for Use]. Merrimack, NH: Atrium Medical Corporation; 2023. AW009603-EN Rev 11.
- Panneton JM, Bismuth J, Gray BH, Holden A. Three-year follow-up of patients with iliac occlusive disease treated with the Viabahn Balloon-Expandable Endoprosthesis. Journal of Endovascular Therapy 2020;27(5):728-736.
- Bismuth J, Gray BH, Holden A, Metzger C, Panneton J; VBX FLEX Study Investigators. Pivotal study of a next-generation balloon-expandable stent-graft for treatment of iliac occlusive disease. Journal of Endovascular Therapy 2017;24(5):629-637. http://journals.sagepub.com/doi/full/10.1177/1526602817720463.
- Holden A, Takele E, Hill A, et al. Long-term follow-up of subjects with iliac occlusive disease treated with the Viabahn VBX Balloon-Expandable Endoprosthesis. Journal of Endovascular Therapy. In press.

INDICATIONS FOR USE IN THE U.S.: The GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis is indicated for the treatment of de novo or restenotic lesions found in iliac arteries with reference vessel diameters ranging from 5 mm–13 mm and lesion lengths up to 110 mm, including lesions at the aortic bifurcation. CONTRAINDICATIONS: Do not use the GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis in patients with known hypersensitivity to heparin, including those patients who have had a previous incident of Heparin-Induced Thrombocytopenia (HIT) type II. Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the market where this product is available. RXOnly
2140878-EN