GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis Case Studies: Reconstructing the aortic bifurcation1
Patient presentation
- 76-year-old male
- History of smoking, hypertension, hyperlipidemia, hypercholesterolemia, coronary artery disease
- Bilateral thigh and calf claudication — Rutherford category 3 (severe claudication)
Clinical challenges
- High-grade, diffuse common iliac stenoses bilaterally
- TASC II Type C lesion
- Severely atheromatous aortoiliac arteries
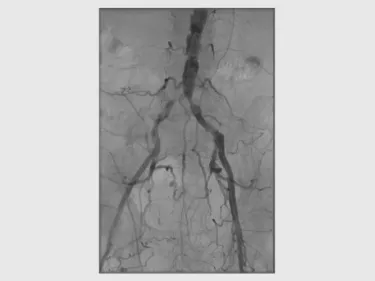
Procedure
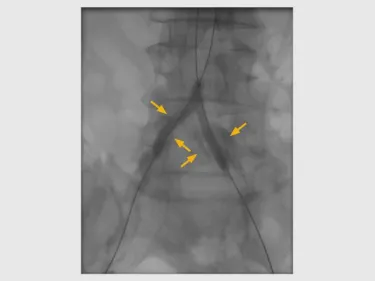
Conservative pre-dilatation to address high-grade stenosis and severe calcification
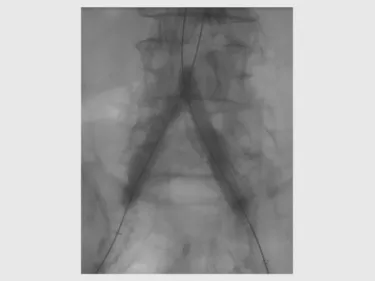
VBX Stent Graft deployment: “kissing” placement reconstructs the aortic bifurcation
Results
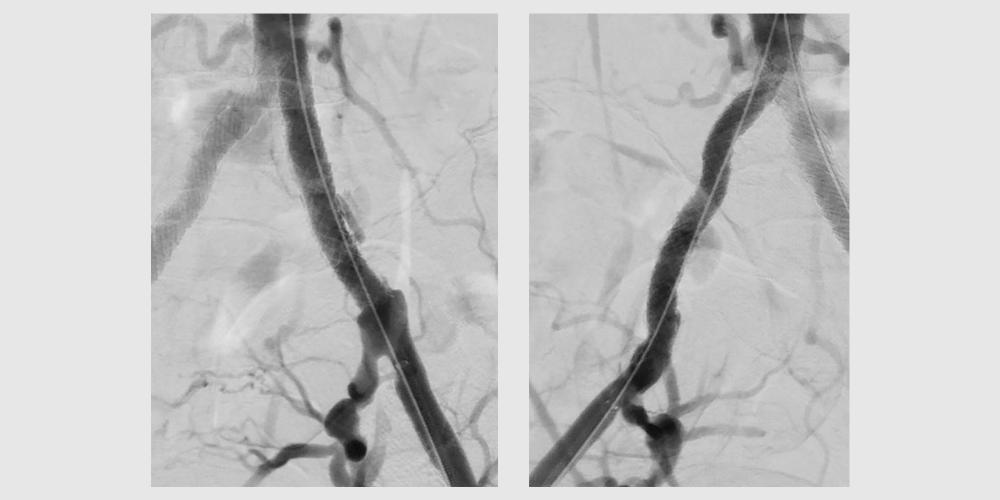
Completion retrograde angiography demonstrates widely patent iliac arteries with
VBX Stent Graft
- Holden A, Merrilees S, Buckley B, Connor B, Colgan F, Hill A. First-in-human experience with the Gore Balloon-Expandable Covered Endoprosthesis in iliac artery occlusive disease. Journal of Endovascular Therapy 2017;24(1):11-18
Procedural outcomes based on usage of legacy GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis. (BXA catalogue numbers).
The outcomes and observations reported are based on individual case experience and the patients treated. The steps described here may not be complete, and are not intended to be a replacement for the Instructions for Use or the education, training and professional judgment of health care providers (HCP). HCPs remain solely responsible for making decisions about patient care and the use of medical technologies.

Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. RXOnly
INDICATIONS FOR USE IN THE U.S.: The GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis is indicated for the treatment of de novo or restenotic lesions found in iliac arteries with reference vessel diameters ranging from 5 mm–13 mm and lesion lengths up to 110 mm, including lesions at the aortic bifurcation. The GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis is also indicated for use with thoracoabdominal and pararenal branched devices indicated with the GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis as a branch component.*
CONTRAINDICATIONS: Do not use the GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis in patients with known hypersensitivity to heparin, including those patients who have had a previous incident of Heparin-Induced Thrombocytopenia (HIT) type II.
* Not applicable to Reduced Profile GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis. (BXB catalogue numbers).
The GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis is not authorized for use with thoracoabdominal and pararenal branched devices indicated with the GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis as a branch component in Canada.