Advancing Care, Minimizing Air

You always seek the best options for patients
We always seek ways to support you
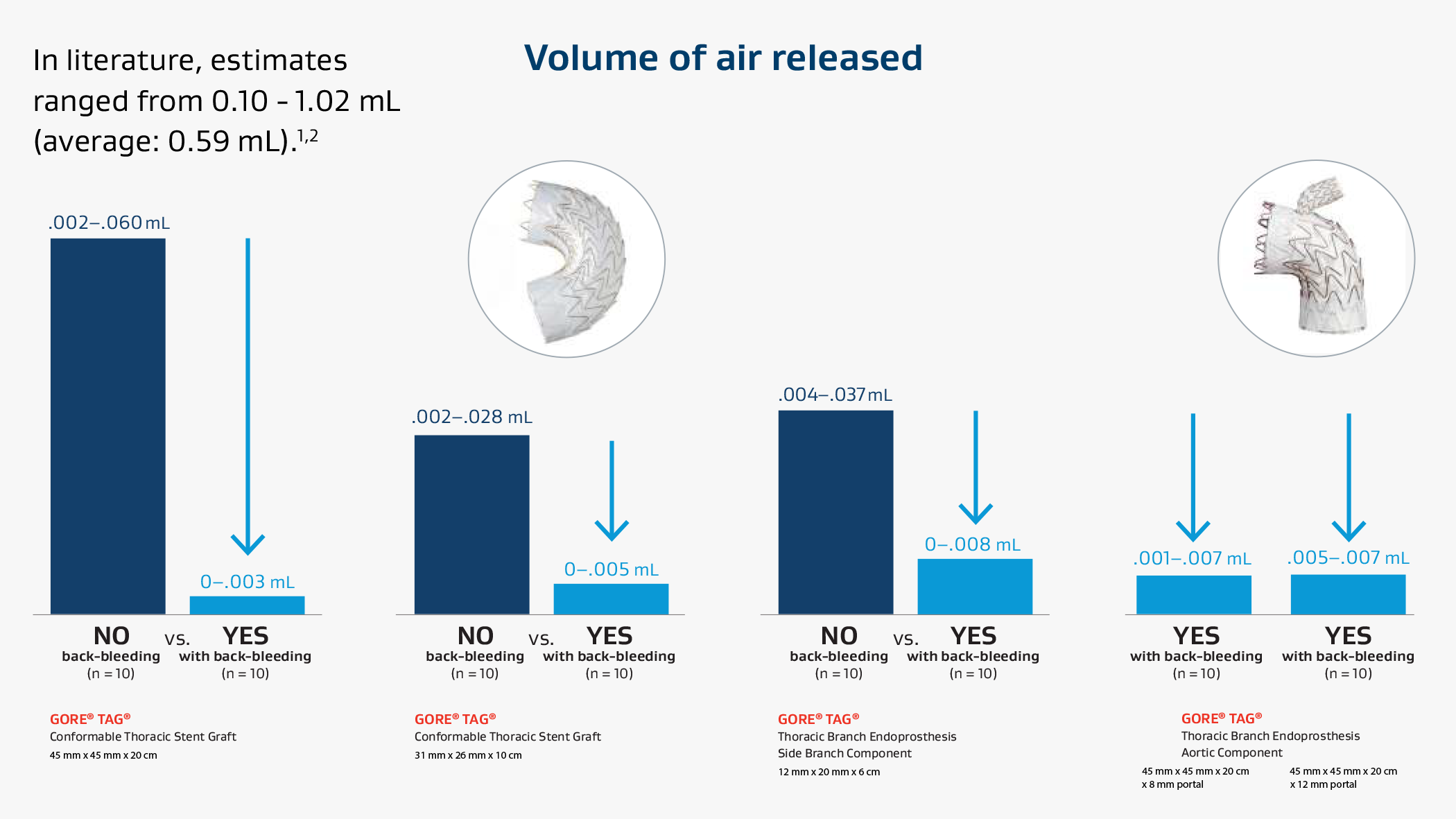
As your Aortic Ally, we listen carefully when we hear conversations around patient outcomes. In recent literature, questions have been raised around the volume of air released by TEVAR endografts. Estimates ranged from 0.10 - 1.02 mL (average: 0.59 mL) based on limited testing using various flushing techniques not included in respective device Instructions for Use.1,2
We’re here to clear the air
When used as recommended, 3-5 Gore TEVAR solutions facilitate a means for minimizing air.*
Distinctive designs enable effective techniques:
- Constraining sleeve: Enables use with the exclusive GORE® DRYSEAL Valve.
- Pressurized valve: Creates a squeegee effect, removing air from the constrained device during insertion.
- Back-bleeding: Pausing insertion allows blood to flow back out of the exposed end of the device, releasing additional air.
Unique TEVAR Systems in Action
Evaluating air volume with clinically relevant testing methods
An integrated approach in action
To evaluate air volume, we developed a clinically relevant test method that simulated recommended use3-5 and physiological conditions.*
- GORE® DRYSEAL Valve inflated with 2.5 mL saline.3
- Back-bleeding technique.4-5
- Devices deployed in blood mimic with mean arterial pressure (67 mmHg).
Across all devices and configurations, recommended use reduced average air volume to minimal levels in testing.
.016 mL
GORE® DRYSEAL
Flex Introducer Sheath
techonology alone
.002 mL
GORE® DRYSEAL
Flex Introducer Sheath
+ back-bleeding technique
Bench-top evaluations are intended to demonstrate relative physical characteristics and may not correlate to clinical results. The test method here was designed to simulate physiological conditions as closely as possible, with the intent to capture relevant data that would demonstrate likely clinical results.
Related to this product
* Data on file 2023; W. L. Gore & Associates, Inc; Flagstaff, AZ.
- Rohlffs F, Tsilimparis N, Saleptsis V, Diener H, Debus ES, Kölbel T. Air embolism during TEVAR: carbon dioxide flushing decreases the amount of gas released from thoracic stent-grafts during deployment. Journal of Endovascular Therapy 2017;24(1):84-88.
- Inci K, Koutouzi G, Chernoray V, Jeppsson A, Nilsson H, Falkenberg M. Air bubbles are released by thoracic endograft deployment: an in vitro experimental study. SAGE Open Medicine. In press.
- GORE® DRYSEAL Flex Introducer Sheath [Instructions for Use]. Flagstaff, AZ: W. L. Gore & Associates, Inc; 2021. MD179444.
- GORE® TAG® Conformable Thoracic Stent Graft [Instructions for Use]. Flagstaff, AZ: W. L. Gore & Associates, Inc; 2021. MD164940.
- GORE® TAG® Thoracic Branch Endoprosthesis (TBE) [Instructions for Use]. Flagstaff, AZ: W. L. Gore & Associates, Inc; 2022. MD184153.
CBAS is a trademark of Carmeda AB, a wholly owned subsidiary of W. L. Gore & Associates, Inc. GORE, Together, improving life, DRYSEAL, TAG and designs are trademarks of W. L. Gore & Associates, Inc.

Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. RXOnly
GORE® DRYSEAL Flex Introducer Sheath.
INDICATIONS FOR USE IN THE U.S.: The GORE® DrySeal Flex Introducer Sheath is intended to be inserted in the vasculature to provide a conduit for the insertion of endovascular devices while minimizing blood loss associated with such insertions.
CONTRAINDICATIONS: There are no known contraindications for this device. Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available.
GORE® TAG® Conformable Thoracic Stent Graft.
INDICATIONS FOR USE IN THE U.S.: The GORE® TAG® Conformable Thoracic Stent Graft is intended for endovascular repair of all lesions of the descending thoracic aorta, including: isolated lesions in patients who have appropriate anatomy, including: adequate iliac/femoral access, aortic inner diameter in the range of 16-42 mm, ≥ 20 mm non-aneurysmal aorta proximal and distal to the lesion; Type B dissections in patients who have appropriate anatomy, including: adequate iliac/femoral access, ≥ 20 mm landing zone proximal to the primary entry tear; proximal extent of the landing zone must not be dissected, diameter at proximal extent of proximal landing zone in the range of 16-42 mm.
CONTRAINDICATIONS: Patients with known sensitivities or allergies to the device materials; patients who have a condition that threatens to infect the graft. Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available.
GORE® TAG® Thoracic Branch Endoprosthesis (TBE).
INDICATIONS FOR USE IN THE U.S.: The GORE® TAG® Thoracic Branch Endoprosthesis is indicated for endovascular repair of lesions of the descending thoracic aorta, while maintaining flow into the left subclavian artery, in patients who have: Adequate iliac/femoral access; Proximal Aortic Landing Zones: For Isolated Lesion Patients: Proximal landing zone cannot be aneurysmal, dissected, heavily calcified or heavily thrombosed; For Dissection Patients: Primary entry tear must be distal to the left subclavian artery and the proximal extent of the landing zone must not be dissected; Aortic inner diameter range 16–42 mm; Proximal segment length (length from distal edge of left subclavian artery to mid left common carotid ostium) of at least 2.0–4.0 cm, depending on Aortic Component selection; Proximal covered length (measured from distal edge of left subclavian artery to distal edge of left common carotid artery ostium) of at least 15–36 mm, depending on Aortic Component selection; For patients with prior ascending aorta or aortic arch repair with surgical graft: at least 2 cm landing zone proximal to the distal anastomosis; Left Subclavian Landing Zone: Landing zone cannot be aneurysmal, dissected, heavily thrombosed and severely tortuous (180 degree turn within the treated length); Left subclavian artery inner diameter of 6–18 mm, depending on Side Branch Portal diameter selected; Left subclavian artery minimum length of 2.5–3.0 cm, depending on Side Branch Portal diameter selected. Distal Landing Zone (Isolated Lesion Patients only): Outer curve length must be ≥ 2 cm proximal to celiac artery; Aortic inner diameter range 16-42 mm; Non aneurysmal, dissected, heavily calcified or heavily thrombosed landing zone; Native Aorta or previously placed GORE® TAG® Conformable Thoracic Stent Graft.
CONTRAINDICATIONS: The GORE® TAG® Thoracic Branch Endoprosthesis is contraindicated in: Patients with known sensitivities or allergies to the device materials [ePTFE (polytetrafluoroethylene), FEP (fluoroethylpropylene), Nitinol (nickel, titanium), Gold, SB Component only – Heparin (CBAS® Heparin Surface)]; Patients who have a condition that threatens to infect the graft; Patients with known hypersensitivity to heparin, including those patients who have had a previous incident of HeparinInduced Thrombocytopenia (HIT) type II. Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available.