Clinical Experience
Eight-month post-op view of GORE® BIO-A® Tissue Reinforcement used for posterior suture-line reinforcement of crural closure.
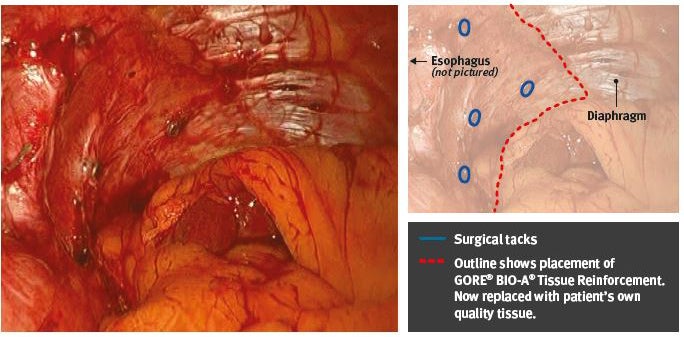
Guided tissue generation with GORE

Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. RXOnly
INDICATIONS FOR USE: The GORE® BIO-A® Tissue Reinforcement is intended for use in the reinforcement of soft tissue. An example of an application where the GORE® BIO-A® Tissue Reinforcement may be used is hernia repair as suture line reinforcement.
CONTRAINDICATIONS: The GORE® BIO-A® Tissue Reinforcement is contraindicated for use in reconstruction of cardiovascular defects. Because GORE® BIO-A® Tissue Reinforcement is absorbable, it is contraindicated for use in patients requiring permanent support from the device.
