GORE® ACUSEAL Vascular Graft
The GORE® ACUSEAL Vascular Graft allows for earlier removal or avoidance of a central venous catheter, providing an alternative for hemodialysis patients.

An immediate vascular access solution, from fistula-first to patient-first.
For hemodialysis patients, a "fistula first" approach has long been the standard. But fistulas can fail, 20 to 60 percent of the time,1-5 subjecting patients to undue hardship and central venous catheter (CVC)-related risks.
That's one reason 2019 Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines6 emphasize the need for a more individualized approach to access modality selection.
When patients are at risk for fistula non-maturation, turn to the GORE® ACUSEAL Vascular Graft – the purpose-built choice for rapid and long-term access for the road ahead. It is ideal for patients who are already on a CVC, or those who need a new access and must achieve cannulation in under two weeks.
It allows for earlier removal or avoidance of a CVC and helps achieve hemodialysis success with reduced access-related procedures and complications8,9 ─ for more confidence at every step.
Immediate cannulation:
Cannulate within 24 hours with the only FDA-cleared vascular access graft with the claim of early cannulation7
Reduced infection and mortality:
5X
lower risk of infection, compared with CVC8
3X
lower risk of mortality, compared with CVC8
Long-term access:
78%
secondary patency demonstrated at 12 months10
Lower total cost of care:
Reduces the cost of treating CVC sepsis by allowing for earlier removal of CVCs.*,11
Designed with your patients in mind
Because dialysis access is personal
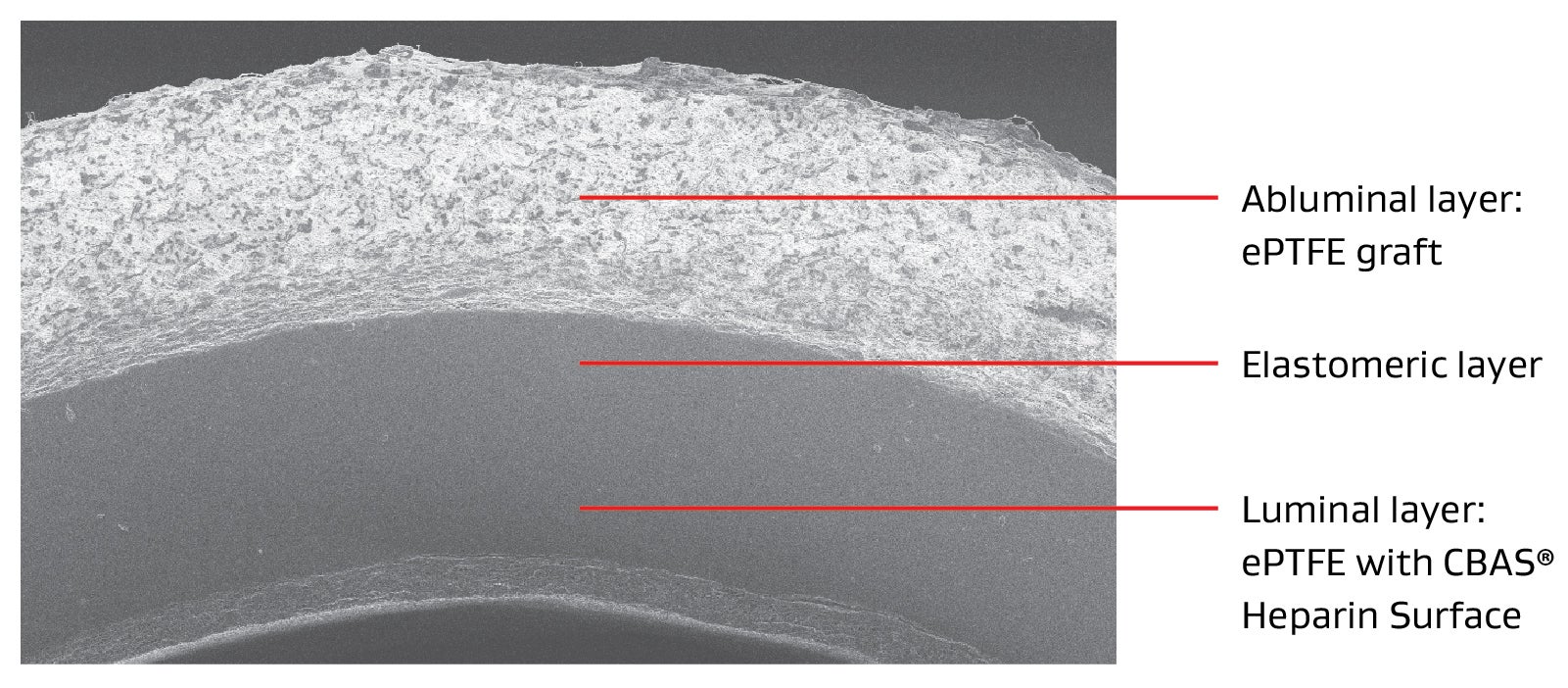
The tri-layer design of the GORE® ACUSEAL Vascular Graft is optimized for early cannulation and provides uncompromised handling.
- Purpose-built for immediate cannulation, designed with an elastomeric middle layer that serves as a low bleed barrier to cannulation needle holes.
- Flexible at curves without kinking.
Reported ability to significantly reduce early thrombosis rates†,13
Reported potential to improve primary patency by up to
20%†,14
78%
clot-free survival reported at one year14
Related products
* Compared with non-immediate cannulation AV grafts.
† Compared with standard non-heparin bonded ePTFE grafts.
- Allon M, Robbin ML. Increasing arteriovenous fistulas in hemodialysis patients: problems and solutions. Kidney International 2002;62(4):1109-1124.
- Allon M, Imrey PB, Cheung AK, et al; Hemodialysis Fistula Maturation (HFM) Study Group. Relationships between clinical processes and arteriovenous fistula cannulation and maturation: a multicenter prospective cohort study. American Journal of Kidney Diseases 2018;71(5):677-689.
- Dember LM, Beck GJ, Allon M, et al; Dialysis Access Consortium Study Group. Effect of Clopidogrel on early failure of arteriovenous fistulas for hemodialysis. A randomized controlled trial. Journal of the American Medical Association 2008;299(18):2164-2171.
- Allon M. Vascular access for hemodialysis patients: new data should guide decision making. Clinical Journal of the American Society of Nephrology 2019;14(6):954-961.
- Wish JB. Catheter last, fistula not-so-first. Journal of the American Society of Nephrology 2015;26(1):5-7.
- Lok CE, Huber TS, Lee T, et al; KDOQI Vascular Access Guideline Work Group. KDOQI Clinical Practice Guideline for Vascular Access: 2019 update. American Journal of Kidney Diseases 2020;75(4)Supplement 2:S1-S164.
- Marketrack: PriceTrack Database. Burlington, MA: Decision Resources Group; 2015 https://decisionresourcesgroup.com. Accessed August 14, 2017.
- Aitken E, Thomson P, Bainbridge L, Kasthuri R, Mohr B, Kingsmore D. A randomized controlled trial and cost-effectiveness analysis of early cannulation arteriovenous grafts versus tunneled central venous catheters in patients requiring urgent vascular access for hemodialysis. Journal of Vascular Surgery 2017;65(3):766-774.
- Al-Balas A, Lee T, Young CJ, Kepes JA, Barker-Finkel J, Allon M. The clinical and economic effect of vascular access selection in patients initiating hemodialysis with a catheter. Journal of the American Society of Nephrology 2017;28(12):3679-3687.
- Glickman M. Early cannulation graft: Acuseal. Journal of Vascular Access 2016;17 (Supplement 1):S72-S74.
- Mohr BA, Trovillion PJ. Economic value of preventing central venous catheter sepsis infections with early cannulation arteriovenous grafts (ECAVGS) compared to non-ecavgs. Presented at the ISPOR 20TH Annual International Meeting; May 16-20, 2015; Philadelphia, PA. Value in Health 2015;18(3):A42 PMD27.
- Roy-Chaudhury P, Kelly BS, Melhem M, et al. Vascular access in hemodialysis: issues, management, and emerging concepts. Clinical Cardiology. 2005;23:249-273.
- Shemesh D, Goldin I, Hijazi J, et al. A prospective randomized study of heparin-bonded graft (Propaten) versus standard graft in prosthetic arteriovenous access. Journal of Vascular Surgery 2015 Jul;62(1)115-22. doi:10.1016/j.jvs.2015.01.056. Epub 2015 Mar12. PMID: 25770987.
- Davidson I, Hackerman C, Kapadia A, Minhajuddib A. Heparin bonded hemodialysis e-PTFE graft result in 20% clot free survival benefit. Journal of Vascular Access 2009 Jul-Sep;10(3):153-6. doi: 10.1177/112972980901000303. PMID:19670166.

Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. RXOnly
INDICATIONS FOR USE IN THE U.S.: GORE® ACUSEAL Vascular Grafts are intended for use as a vascular prosthesis in patients requiring vascular access.
INDICATIONS FOR USE IN CANADA: GORE® ACUSEAL Vascular Graft is intended for use as a vascular prostheses in patients requiring vascular access. GORE® ACUSEAL Vascular Graft is indicated for patients who require arteriovenous hemodialysis access in the upper or lower extremities for End Stage Renal Disease (ESRD).
CONTRAINDICATIONS: DO NOT use the GORE® ACUSEAL Vascular Graft in patients with known hypersensitivity to heparin, including those patients who have had a previous incident of Heparin-Induced Thrombocytopenia (HIT) type II. DO NOT use GORE® ACUSEAL Vascular Grafts as a patch. If cut and used as a patch, GORE® ACUSEAL Vascular Grafts may lack adequate transverse strength.


