Trusted closure performance at six months*,1,2

| Characteristics of complex ASDs | |
|---|---|
| ASD Category | Anatomical Characteristics |
| Complex |
|
No retro-aortic rim requirements2
Gore ASSURED Clinical Study:
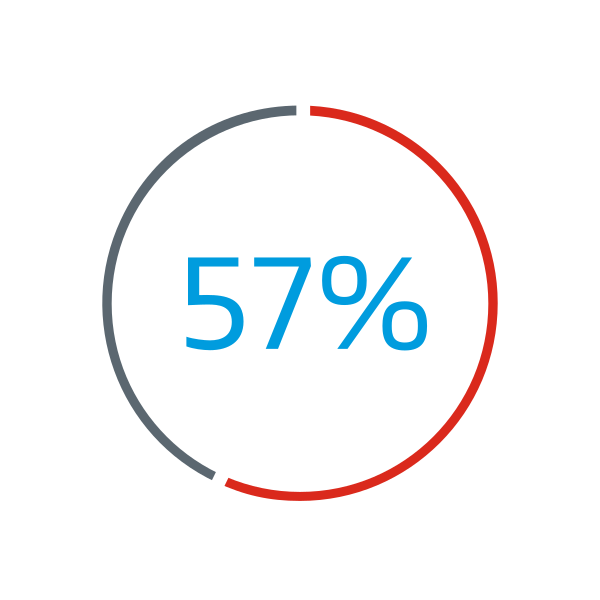
of patients enrolled in the Gore ASSURED Clinical Study were reported to have deficient retro-aortic rims (< 5 mm)1

effective closure at six months*,1,2
GORE® CARDIOFORM ASD Occluder
The only ASD occluder with no warnings, potential adverse events or precautions related to cardiac erosions or use in patients with deficient retro-aortic rims.†,1,2,4,5
Download brochure for more information
Case examples
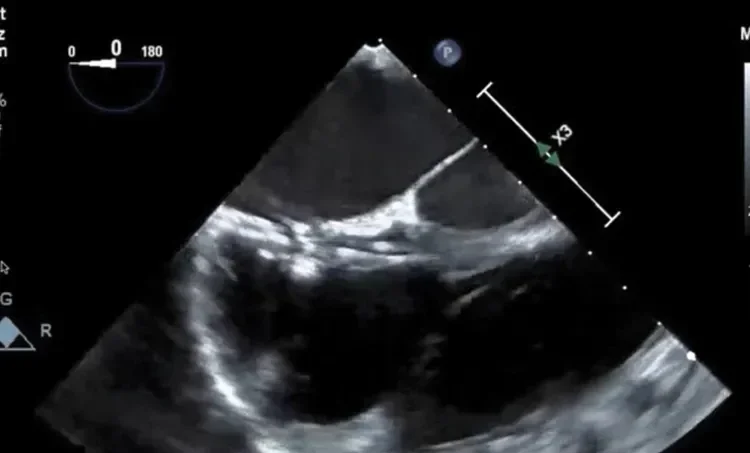
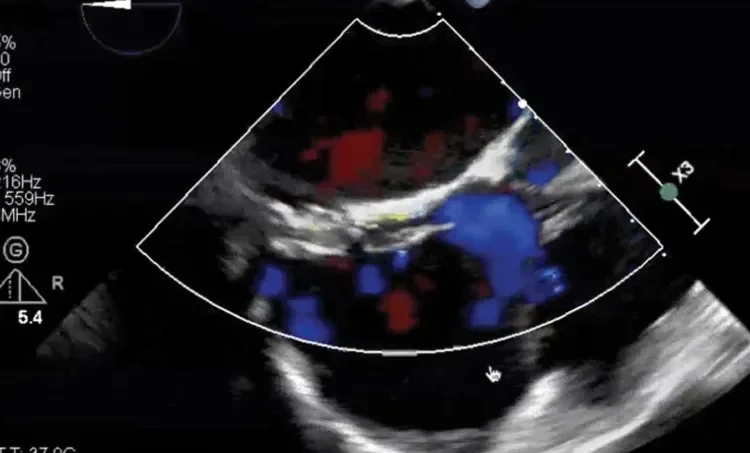
Deficient retro-aortic rim < 5 mm
Large ASD with deficient posterior-inferior rim
Multiple defects with atrial septal aneurysm (ASA)
See why we are a leader in safety
* Closure success defined as completely occluded or clinically insignificant shunt as determined by the Echo Core Lab at the six-month evaluation among subjects with technical success.2
† Deficient retro-aortic rim was defined as a retro-aortic rim measuring less than or equal to 5 mm on any view on echocardiogram.
1. Sommer RJ, Love BA, Paolillo JA, et al.; ASSURED Investigators. ASSURED clinical study: new GORE® CARDIOFORM ASD Occluder for transcatheter closure of atrial septal defect. Catheterization & Cardiovascular Interventions 2020;95(7):1285-1295.
2. GORE® CARDIOFORM ASD Occluder [Instructions for Use]. Flagstaff, AZ.: W. L. Gore & Associates, Inc; 2023. MD190349.
3. Hijazi ZM, Feldman T, Mustafa H, et al. Transcatheter Closure of ASDs and PFOs: A Comprehensive Assessment. 1st Edition. Cardiotext Publishing. 2010.
4. AMPLATZER™ Multifenestrated Septal Occluder – “Cribriform” [Instructions for Use]. Plymouth, MN: Abbott Medical; 2022. ARTEN600196098 B.
5. AMPLATZER™ Septal Occluder [Instructions for Use]. Plymouth, MN: Abbott Medical; 2022. ARTEN600196097 B.

INDICATIONS FOR USE IN THE U.S.: The GORE® CARDIOFORM ASD Occluder is a permanently implanted device indicated for the percutaneous, transcatheter closure of ostium secundum atrial septal defects (ASDs).
CONTRAINDICATIONS: The GORE® CARDIOFORM ASD Occluder is contraindicated for use in patients: Unable to take anti-platelet or anticoagulant medications such as aspirin, heparin or warfarin; with anatomy where the GORE® CARDIOFORM ASD Occluder size or position would interfere with other intracardiac or intravascular structures, such as cardiac valves or pulmonary veins; with active endocarditis, or other infections producing bacteremia, or patients with known sepsis within one month of planned implantation, or any other infection that cannot be treated successfully prior to device placement; with known intracardiac thrombi.
Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. Rx only
241367165-EN