
GORE® SYNECOR Biomaterial
Gore Core Technology
Permanent strength with a lightweight profile*
Choose material engineered to be reliably lasting and deliver permanent strength
Help your patients and yourself when bridging a hernia or soft tissue defect. Gore has applied its core technology (PTFE fluoropolymers/PGA:TMC) to solutions for hernia and abdominal wall reconstruction patients who are even more at risk for hernia recurrence and wound healing complications.
High Strength* Gore PTFE Technology
GORE® SYNECOR Biomaterial is designed to provide permanent strength when bridging a hernia or soft tissue defect.
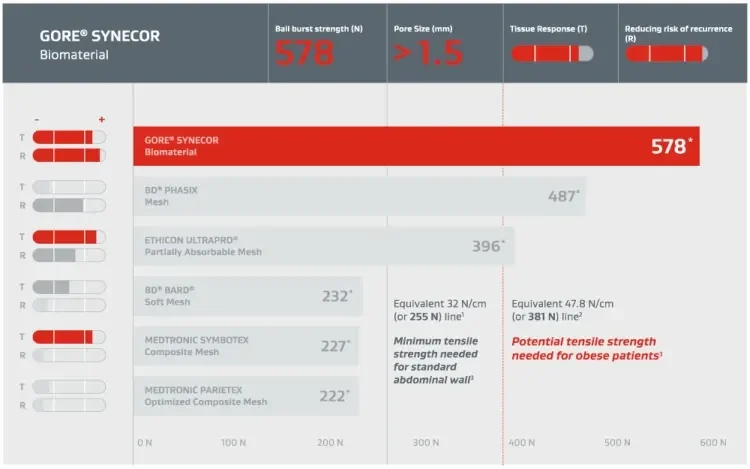
All listed products have been cleared or approved for use in abdominal wall by respective regulatory agencies. Indications may differ by region. Check product Instructions for Use for complete information.
Today’s high-risk patients need more from you. Give them more with GORE® SYNECOR Biomaterial.
* Data on file 2015; W. L. Gore & Associates, Inc; Flagstaff, AZ.
- Klinge U, Klosterhalfen B, Conze J,et al. Modified mesh for hernia repair that is adapted to the physiology of the abdominal wall. European Journal of Surgery 1998;164(12):951-960.
- Zhu LM, Schuster P, Klinge U. Mesh implants: an overview of crucial mesh parameters. World Journal of Gastrointestinal Surgery 2015;7(10) 226-236.
- ATSM D3787-16, Standard Test Method for Bursting Strength of Textiles - Constant-Rate-of-Traverse (CRT0 Ball Burst Tests, ASTIM International, West Conshohocken, PA, 2016, www.astm.org.
BD, BARD and PHASIX are trademarks of Becton, Dickinson and Company.
ETHICON and ULTRAPRO are trademarks of Ethicon, Inc., a Johnson and Johnson Company.
MEDTRONIC, PARIETEX and SYMBOTEX are trademarks of Medtronic, Inc.

Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. RXOnly
231201681-EN