Product Value—GORE® SYNECOR Biomaterials
Quality outcomes start with quality materials
 The GORE® SYNECOR Biomaterials offer permanent strength with rapid* tissue ingrowth to promote healing and are designed to minimize complications in the repair of hernias and abdominal wall soft tissue deficiencies.
The GORE® SYNECOR Biomaterials offer permanent strength with rapid* tissue ingrowth to promote healing and are designed to minimize complications in the repair of hernias and abdominal wall soft tissue deficiencies.
Projected costs for hernia repair in the U.S.

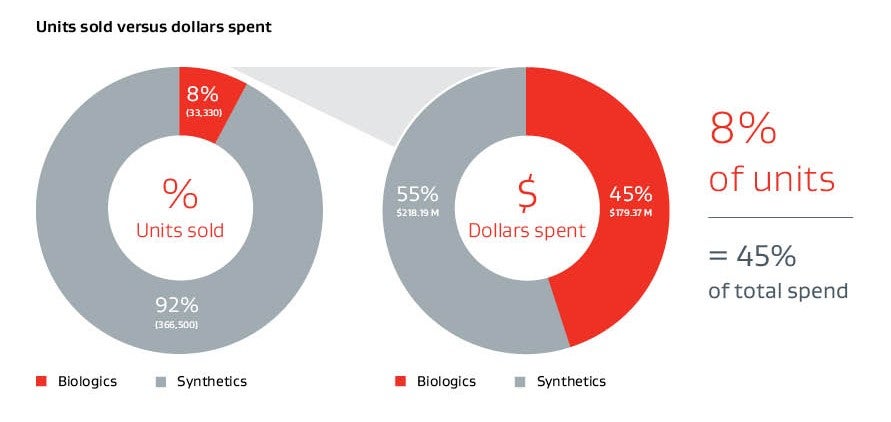
Typical ventral hernia mesh spend for U.S. hospitals 20194
Reducing Recurrence Rate
Data indicates the risk of hernia recurrence is a leading factor for physicians in choosing a mesh;* risk of complications and comorbid conditions are some of the patient characteristics that drive this choice.
Ventral hernia repairs (VHRs) continue to rise in incidence and cost. By reducing recurrence rate alone, a cost savings of the U.S. $32 million dollars for each 1% reduction in operations would result.5

GORE® SYNECOR Biomaterial is less than half the cost of leading biologics and the cost is favorable compared to other biosynthetics.6 Based on patient selection criteria, clinicians may utilize GORE® SYNECOR Biomaterial as an alternative.
Compared to biologics, GORE® SYNECOR Biomaterials offer permanent strength that may lead to fewer hernia recurrences and better quality outcomes by:
- Enabling single-stage durable repair for challenging hernia cases, and may reduce overall cost.
- Lowering costs to use compared to biologic and long-term resorbable products of similar size.6
- Lightweight mesh clinical studies demonstrating a potential for reduced re-interventions and fewer complications for complex, high-risk hernia repair.7,8
- Allowing for a single surgery with potential for faster recovery and fewer complications versus a staged-repair requiring multiple surgeries.

Connect with one of our Corporate Account Directors to learn more about the value Gore can deliver to your healthcare institution.
Contact us >
INDICATIONS FOR USE: The GORE® SYNECOR Intraperitoneal Biomaterial is intended for use in the repair of hernias and abdominal wall or thoracic wall soft tissue deficiencies that may require the addition of non-absorbable reinforcing or bridging material.
CONTRAINDICATIONS: Not for reconstruction of cardiovascular defects.
INDICATIONS FOR USE: The GORE® SYNECOR Preperitoneal Biomaterial is intended for use in the repair of hernias and abdominal wall soft tissue deficiencies that may require the addition of a non-absorbable reinforcing or bridging material.
CONTRAINDICATIONS: Not for reconstruction of cardiovascular defects.
Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. RXOnly

* Data on file 2015; W. L. Gore & Associates, Inc; Flagstaff, AZ.
- Luijendijk RW, Hop WC, van den Tol MP, et al. A comparison of suture repair with mesh repair for incisional hernia. New England Journal of Medicine 2000;343(6):392-398.
- Saleh S, Plymale MA, Davenport DL, Roth JS. Risk-assessment score and patient optimization as cost predictors for ventral hernia repair. Journal of the American College of Surgeons 2018;226(4):540-546.
- Fischer JP, Basta MN, Mirzabeigi MN, et al. A risk model and cost analysis of incisional hernia after elective abdominal surgery based on 12,373 cases. the case for targeted prophylactic intervention. Annals of Surgery 2016;263(5):1010-1017.
- © 2022 Millennium Research Group, Inc. All rights reserved. Reproduction, distribution, transmission or publication is prohibited. Reprinted with permission.
- Poulose BK, Shelton J, Phillips S, et al. Epidemiology and cost of ventral hernia repair: making the case for hernia research. Hernia 2012;16(2):179-183.
- Nisiewicz MJ, Plymale MA, Davenport DL, et al. Validation and extension of the ventral hernia repair cost prediction model. Journal of Surgical Research 2019;244:153-159.
- Carbonell A, Cobb W, Doerhoff C, et al. Clinical Quality Improvement (CQI): a post market method to evaluate a new mesh for ventral/incisional hernia repair. Presented at the Abdominal Wall Reconstruction Conference; June 8-10, 2017; Washington, D.C.
- Grimsley L, Forman B, Carbonell A, et al. Evaluating the real world use of a new hybrid hernia mesh. Presented at the 2018 International Hernia Congress, March 12-15, 2018, Miami, FL. Hernia 2018;22(1)Supplement:S178. P-1316.
22455252-EN

