
GORE® VIABAHN® Device family
Join the next generation of care in the treatment of aortoiliac occlusive disease
The latest generation of covered stent grafts
GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis (VBX Stent Graft)
Decide with data
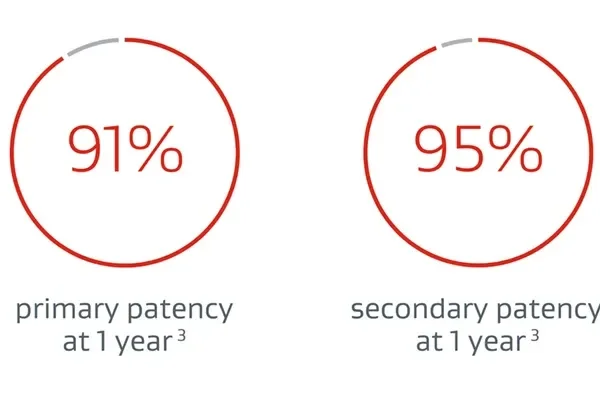
The Gore VBX FLEX Clinical Study 3-year follow-up found the VBX Stent Graft to be a robust and durable treatment option for AIOD.1 Now, sustained patient benefit and durability are demonstrated through 5 years.
Investigator Insights
Andrew Holden, M.D. discusses the background, outcomes and implications of this 5-year long-term data.
Physician-initiated 5-year VBX Stent Graft follow-up
Objective and methodology2
- This physician-initiated study enrolled 59 patients from 3 participating centers that were representative of the VBX FLEX Study 3-year follow-up cohort. Twenty-eight patients completed the 5-year follow-up.
- The primary durability endpoint was long-term primary patency.
DURABLE CLINICAL OUTCOMES THROUGH 5 YEARS2:
SUSTAINED PATIENT BENEFIT THROUGH 5 YEARS2:
The case for long-term durable clinical outcomes:
Case study 1: Treating severe aortoiliac occlusive disease (AIOD) at the aortic bifurcation
Case study 2: Restoring flow to a patient with stenosis at the aortoiliac bifurcation
Case study 3: Restoring flow in a patient with severe claudication and focal iliac calcification

GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface†
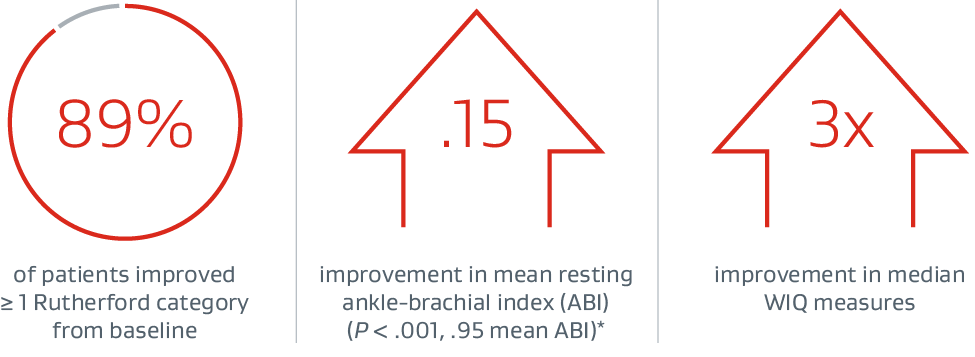
Proven patency with unmatched versatility‡

Visit the VIABAHN® Device product page. >
VIABAHN® Device outcomes in iliac occlusive disease
Prospective, multicenter, single-arm study
53 patients (61 limbs) with iliac artery occlusion or stenosis (Mean lesion length: 6.9 cm; 48% of limbs in the external iliac artery)
* (P < .001) Statistically significant change from pre-procedure.
† As used by Gore, Heparin Bioactive Surface refers to Gore's proprietary CBAS® Heparin Surface.
‡ Across indication inclusivity, and configuration breadth/capability of balloon expandable covered stents.
- Panneton JM, Bismuth J, Gray BH, Holden A. Three-year follow-up of patients with iliac occlusive disease treated with the Viabahn Balloon-Expandable Endoprosthesis. Journal of Endovascular Therapy 2020;27(5):728-736.
- Holden A, Takele E, Hill A, et al. Long-term follow-up of subjects with iliac occlusive disease treated with the Viabahn VBX Balloon-Expandable Endoprosthesis. Journal of Endovascular Therapy. In press.
- Lammer J, Dake MD, Bleyn J, et al. Peripheral arterial obstruction: prospective study of treatment with a transluminally placed self-expanding stent graft. Radiology 2000;217(1):95-104.

INDICATIONS FOR USE IN THE U.S.: The GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis is indicated for the treatment of de novo or restenotic lesions found in iliac arteries with reference vessel diameters ranging from 5 mm–13 mm and lesion lengths up to 110 mm, including lesions at the aortic bifurcation.
CONTRAINDICATIONS: Do not use the GORE® VIABAHN® VBX Balloon Expandable Endoprosthesis in patients with known hypersensitivity to heparin, including those patients who have had a previous incident of Heparin-Induced Thrombocytopenia (HIT) type II. Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the market where this product is available. RXOnly
22703753-EN