GORE® ENFORM Biomaterial
25% stronger than BD® PHASIX® Mesh*,†,7
ZERO complete mesh removals due to infection reported‡,§
Fully absorbable mesh solution for ventral hernia repair that delivers a strong, rapid repair with native tissue in 6 to 7 months — leaving behind nothing but a strong repair with a low risk of infection.*,8
STRENGTH
Absorbable devices
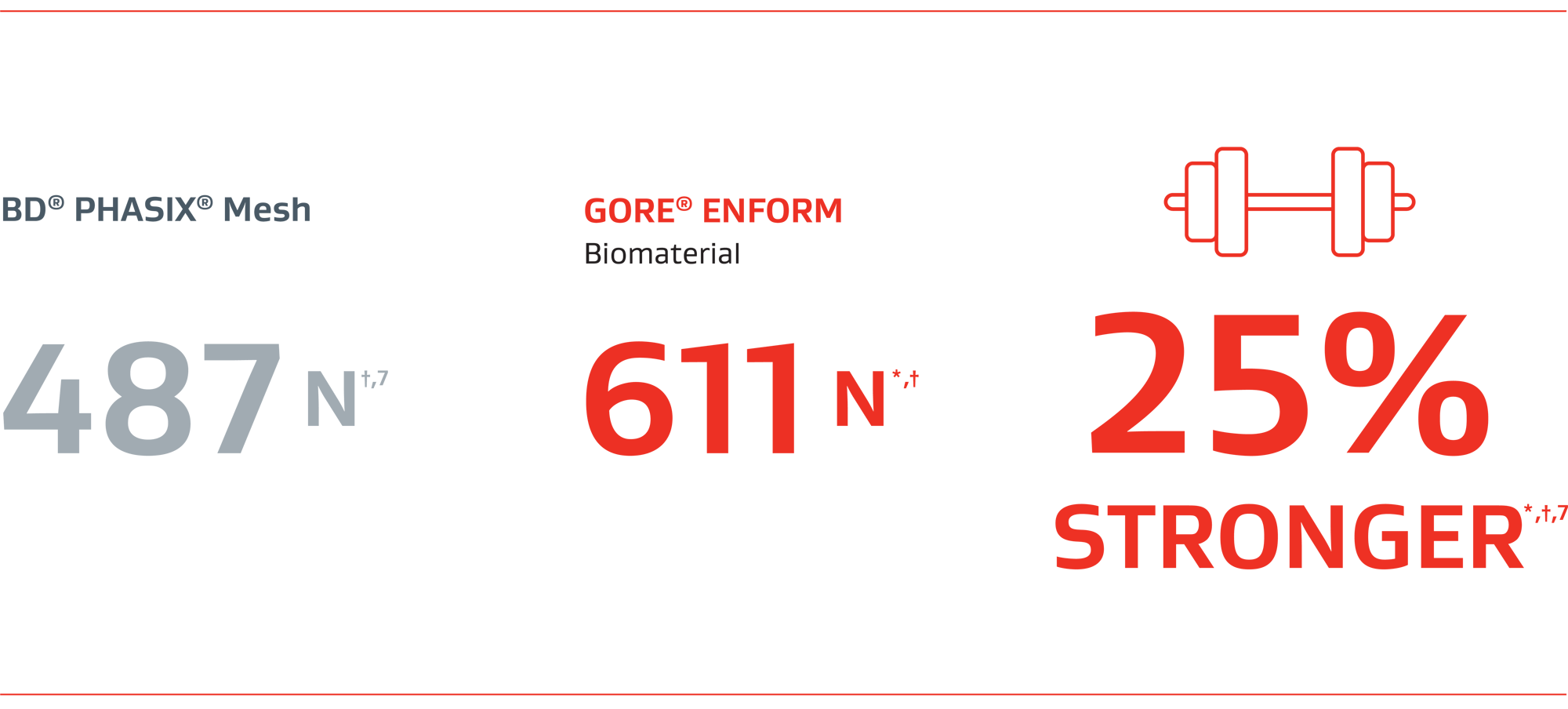
Ball burst strength‡ (N)
Months until fully replaced by healthy tissue
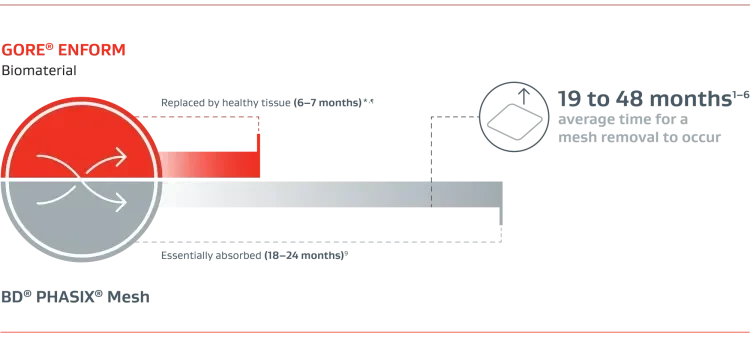
GORE® ENFORM Biomaterial

40% more strength than a suture only repair at 4 months*

Tailor to match your preferred technique with soft and conformable material

Type 1 collagen generation in 6 to 7 months for a strong repair with healthy native tissue*,8

Low inflammatory response and minimized seroma*

6x more vascularity than BD® PHASIX® Mesh at 30 days after implantation*

12,000 patient lives positively impacted*

Gore ventral hernia solutions balance strength with a low rate of complications for your high-risk patients which is backed by*,**:
- 15 years of extensive clinical literature in soft tissue reinforcement*
- Over 40 publications††
- More than 4,000 cases in literature††
Is a permanent device what your high-BMI, co-morbid patients need?
GORE® SYNECOR Biomaterial is 2.5x stronger than BD® BARD® Soft Mesh and 14% stronger than BD® VENTRALIGHT® ST Mesh with zero fractures reported.*,II,‡‡,§§,10-12
See it in action.
* Data on file, W. L. Gore & Associates, Inc; Flagstaff, AZ.
† Out-of-the-box strength.
‡ A literature search was performed by an Information Specialist in January 2024 using the EMBASE® Database and DIMENSIONS Database. Key words/phrases are on file.
§ No confirmed reports of complete mesh removals due to infection reported.
II Bench-top evaluations are intended to demonstrate relative physical characteristics and may not correlate to clinical results.
¶ In a preclinical study.
** GORE® BIO-A® Tissue Reinforcement, GORE® ENFORM Biomaterial, GORE® SYNECOR Biomaterial.
†† A literature search was performed by an Information Specialist in 2024 using the DIMENSIONS® Database, EMBASE® Database, and MEDLINE® on DIALOG® Database. Key words/phrases are on file.
‡‡ No confirmed fractures reported.
§§ Includes only the permanent component of these products.
1. Kao AM, Arnold MR, Augenstein VA, Heniford BT. Prevention and treatment strategies for mesh infection in abdominal wall reconstruction. Plastic & Reconstructive Surgery 2018;142(3)Supplement:149S155S.
2. Warren JA, Love M, Cobb WS, Beffa LR, Couto FJ, Hancock BH, Morrow D, Ewing JA, Carbonell AM. Factors affecting salvage rate of infected prosthetic mesh. American Journal of Surgery 2020;220(3):751-756.
3. Birolini C, de Miranda JS, Tanaka EY, Utiyama EM, Rasslan S, Birolini D. The use of synthetic mesh in contaminated and infected abdominal wall repairs: challenging the dogma-a long-term prospective clinical trial. Hernia 2020;24(2):307-323.
4. Jin C, Shen Y, Chen J. Laparoscopic evaluation and management of 47 patients with late-onset mesh infection after inguinal hernioplasty. Hernia 2020;24(2):381-385.
5. Arnold MR, Kao AM, Otero J, et al. Mesh fistula after ventral hernia repair: what is the optimal management? Surgery 2020;167(3):590-597.
6. Harris HW, Primus F, Young C, et al. Preventing recurrence in clean and contaminated hernias using biologic versus synthetic mesh in ventral hernia repair: The PRICE randomized clinical trial. Annals of Surgery 2021;273(4):648-655
7. Deeken CR, Matthews BD. Characterization of the mechanical strength, resorption properties, and histologic characteristics of a fully absorbable material (Poly-4-hydroxybutyrate-PHASIX Mesh) in a porcine model of hernia repair. ISRN Surgery 2013;2013:238067.
8. Zemlyak A, Colavita P, Tsirline V, et al. Absorbable glycolic acid/trimethylene carbonate synthetic mesh demonstrates superior in-growth and collagen deposition. Presented at the 4th Annual European Plastic Surgery Research Council (EPSRC) Meeting; August 23-26, 2012; Hamburg, Germany. Plastic & Reconstructive Surgery 2012;130(Supplement 2S):493. Abstract LOP59.
9. Phasix™ Mesh Fully Resorbable Implant for Soft Tissue Reconstruction [Instructions for Use]. Warwick, RI: Davol Inc. Subsidiary of C. R. Bard, Inc; 2016. PK3799200. 1611R. DAV/PHSX/0915/0062(3).
10. Olson TB. Competitive Hernia Device Strength Evaluation. Flagstaff, AZ: W. L. Gore & Associates, Inc; 2016. [Work plan]. WP108484
11. Olson TB. Ventralight ST Strength after 14 and 28 day Degradation of Absorbable Components. Flagstaff, AZ: W. L. Gore & Associates, Inc; 2016. [Work plan]. WP108781.
12. W. L. Gore & Associates, Inc. Plexus Knit PQ Validation Report. Flagstaff, AZ: W. L. Gore & Associates, Inc; 2022. [Validation Report]. MD145325. Rev 5.

Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. RXOnly
BD, BARD, PHASIX and VENTRALIGHT are trademarks of C.R. Bard, Inc.
DIMENSIONS is a trademark of Digital Science & Research Solutions Inc.
EMBASE is a trademark of Elsevier Limited.
DIALOG is a trademark of Proquest LLC.
MEDLINE is a trademark of The National Library of Medicine.
24GP1031-EN01