Proven procedural and clinical success†

restoration of lumen diameter1
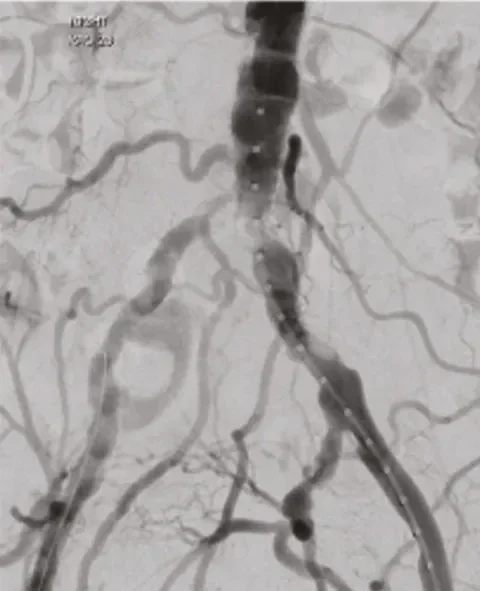
Before
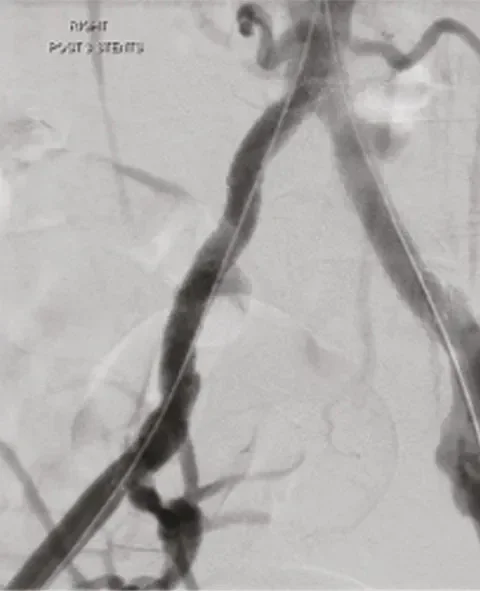
After
≤ 30% residual stenosis due to high radial strength, even in highly calcified and non-compliant lesions.
The Gore VBX FLEX Clinical Study is a prospective, multicenter, single arm study of 134 patients with complex aortoiliac occlusive disease (32.1% TASC II C and D, 42.5% kissing stent).
In the study, 234 devices were delivered; 50% bilateral treatment, 18% contralateral deliveries and predilitation was not required.

maintenance of stent length1
Median length change was 0 mm, predeployed to final implant
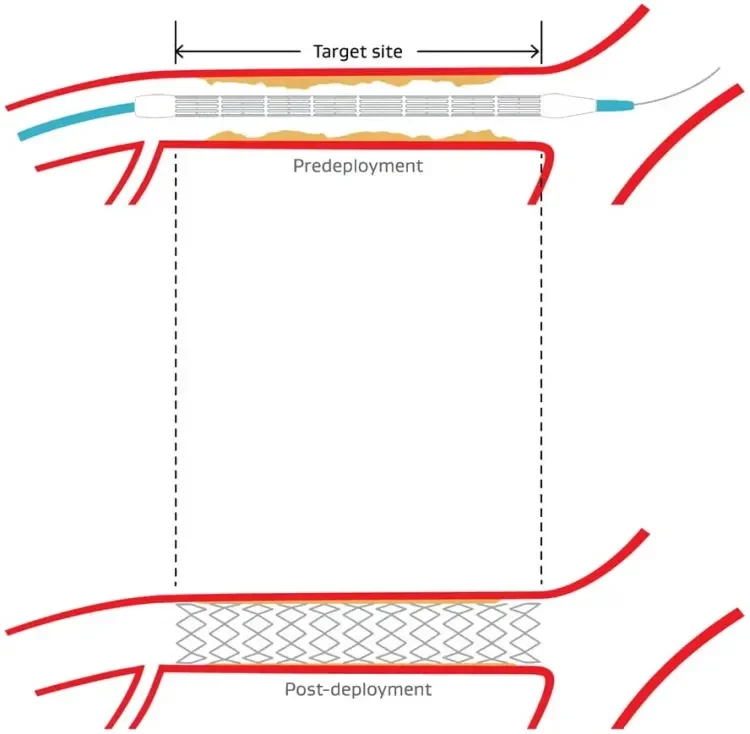

stent delivery
- 100% delivery to target lesion with no device dislodgement1
- 100% stent retention1
- 100% deployment at the target site1
Proven patency and patient benefit†
5-year outcomes
VBX Stent Graft durability through 5 years assessed in a physician-initiated study that enrolled 59 patients from 3 participating centers representative of the VBX FLEX Study cohort.
Clinically proven results*,2

Primary patency per lesion

Primary assisted patency per lesion

fTLR per subject
Additional patient benefits versus baseline*,2

Improvement in mean resting ABI (from .76 to .95) [P < .001]‡
Improvement in median WIQ measures

of evaluated (n = 28) patients improved ≥ 1 Rutherford category from baseline§
3-year outcomes

Freedom from target lesion revascularization (fTLR)3

Improvement in mean resting ankle-brachial index (ABI) (P < .001, .93 mean ABI)‡,3
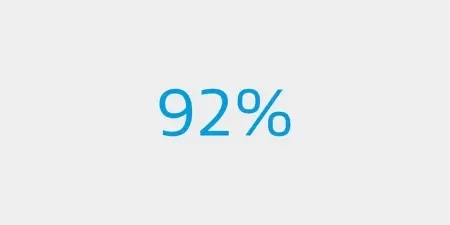
of patients improved ≥ 1 Rutherford category versus baseline3
1-year outcomes

Primary patency3

Primary patency in TASC C&D lesions at 1 year3

Secondary patency3
* Across indications and configurations of covered stents.
† Based on prior clinical data. New evaluation of reduced profile delivery is underway.
‡ (P < .001) Statistically significant change from pre-procedure.
§ 59 subjects participated and 28 were available through the end of the study at 5-year follow-up.
- Bismuth J, Gray BH, Holden A, et al; VBX FLEX Study Investigators. Pivotal study of a next-generation balloon-expandable stent-graft for treatment of iliac occlusive disease. Journal of Endovascular Therapy 2017;24(5):629-637. http://journals.sagepub.com/doi/full/10.1177/1526602817720463
- Holden A, Takele E, Hill A, et al. Long-Term Follow-up of Subjects With Iliac Occlusive Disease Treated With the Viabahn VBX Balloon-Expandable Endoprosthesis. Journal of Endovascular Therapy. 2023;0(0). doi:10.1177/15266028231165723
- Panneton JM, Bismuth J, Gray BH, Holden A. Three-year follow-up of patients with iliac occlusive disease treated with the Viabahn Balloon-Expandable Endoprosthesis. Journal of Endovascular Therapy 2020;27(5):728-736. https://journals.sagepub.com/doi/10.1177/1526602820920569
![]()
Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. RXOnly
24PL2171-EN01
