Understanding Venous Disease
How you can help yourself or others living with inferior vena cava syndrome (IVCS) disease and iliofemoral venous obstruction
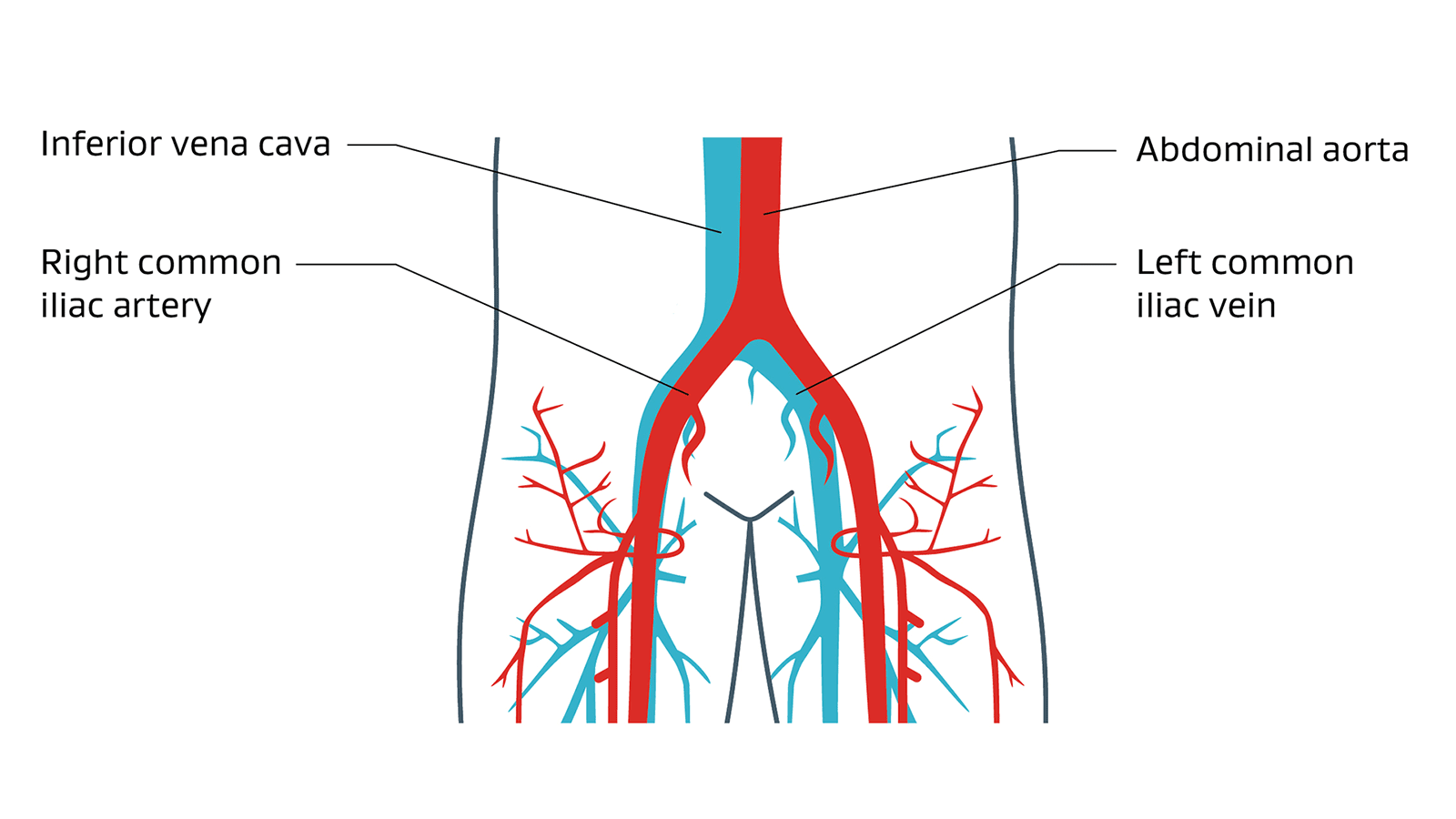
Two types of venous disease
Inferior vena cava syndrome, or IVCS, refers to a sequence of signs and symptoms related to obstruction or compression of the inferior vena cava (IVC), a large vein that carries blood from the lower and middle part of the body back to the heart.1
Iliofemoral venous obstruction is a partial or complete blockage of the iliac and femoral veins (in the pelvis and thigh). This type of obstruction sometimes extends from the common femoral vein (CFV) in the thigh up through the inferior vena cava.2
Venous disease signs and symptoms
Venous disease affects about 1 in 3 adults.3 There are many types of venous diseases, as well as many signs and symptoms. These signs and symptoms can include3,4:
- Leg swelling
- Leg pain
- Leg skin changes
- Leg ulcers (not healing)
If you notice any of these symptoms, please contact your doctor who may refer you to a vascular specialist for a complete diagnosis.
Current venous disease treatments
For those with venous disease, a physician and/or surgeon may recommend 1 or more common treatment options, including but not limited to3:
- Compression stockings
- Anticoagulation (blood-thinning medications to prevent blood clots)
- Thrombolytics (medications to dissolve a blood clot)
- Thrombectomy (surgery to remove a blood clot or clots)
- Angioplasty (a procedure that inflates a balloon to open up the vein) plus stent placement
- Stent placement is a procedure that places a tubular support inside a blood vessel to aid healing or relieve an obstruction
- Venous stenting (placing a stent inside the vein)
Potentially helpful lifestyle modifications
Health care providers may also recommend lifestyle modifications instead of or as complement to other treatments, such as3:
- Elevating the legs
- Exercise improvements
Talk to your doctor about appropriate treatment options.
Clinical studies enrolling

The Investigational GORE® VIAFORT Vascular Stent Iliofemoral Venous Study
For treatment of symptomatic iliofemoral venous obstruction, a clinical study will evaluate the performance, safety and efficacy of using the investigational GORE® VIAFORT Vascular Stent.

The Investigational GORE® VIAFORT Vascular Stent IVC Study
For treatment of symptomatic IVC obstruction with or without combined iliofemoral venous obstruction, a clinical study will evaluate the performance, safety and efficacy of using the investigational GORE® VIAFORT Vascular Stent.
See details at clinicaltrials.gov
CAUTION
lnvestigational device. Limited by Federal (or United States) law to investigational use.
Find a study location
California
Leland Stanford Junior University
300 Pasteur Drive
Stanford, CA 94305
Principal Investigator: Andrew Kesselman, M.D.
Colorado
Advanced Heart and Vein Center
805 E 144th Ave Suite 100
Thornton, CO 80023
Principal Investigator: Ehrin Armstrong, M.D.
Connecticut
Vascular Care Group
330 Boston Post Rd, Suite 240
Darien, CT 06820
Principal Investigator: Paul Gagne, M.D.
Yale University
300 Cedar Street
New Haven, CT 06519
Principal Investigator: Cassius Chaar, M.D.
District of Columbia (D.C.)
MedStar Washington Hospital Center
110 Irving Street Northwest
Washington, DC 20010
Principal Investigator: Steven Abramowitz, M.D.
Florida
Manatee Hospital
250 2nd Street, Suite 4D
Bradenton, FL 34208
Principal Investigator: Jay Mathews, M.D.
Illinois
Northwestern University
676 N St. Clair Street, Suite 1400
Chicago, IL 60611
Principal Investigator: Kush Desai, M.D.
Massachusetts
Massachusetts General Hospital
55 Fruit Street
Boston, MA 02114
Principal Investigator: Julianne Stoughton, M.D.
Michigan
University of Michigan
Frankel Cardiovascular Center
1425 E Ann St
Ann Arbor, MI 48109
Principal Investigator: William Sherk, M.D.
New Jersey
Englewood Health
350 Engle St
Englewood, NJ 07631
Principal Investigator: Steven Elias, M.D.
New York
Mount Sinai Health System
1 Gustave L. Levy Place
New York, NY 10029
Principal Investigator: Windsor Ting, M.D.
St Peters Health Partners
315 S. Manning Blvd.
Albany, NY 12208
Principal Investigator: Kathleen Ozsvath, M.D.
Stony Brook University Medical Center
101 Nicolls Road
Stony Brook, NY 11794
Principal Investigator: Angela Kokkosis, M.D.
North Carolina
Atrium Health-Sanger Heart and Vascular Institute
1237 Harding Place, KMOB I, Suite 5100
Charlotte, NC 28204
Principal Investigator: Erin Murphy, M.D.
Division of Vascular Surgery at UNC Chapel Hill
3024 Burnett Womack CB7212
Chapel Hill, NC 27599
Principal Investigator: Katharine McGinigle, M.D.
NC Heart and Vascular Research
4414 Lake Boone Trail, Suite 409
Raleigh, NC 27607
Principal Investigator: Robert Mendes, M.D.
Ohio
Cleveland Clinic
9500 Euclid Ave
Cleveland, OH 44195
Principal Investigator: Jon Quatromoni, M.D.
TriHealth - Good Samaritan Hospital
375 Dixmyth Ave
Cincinnati, OH 45220
Principal Investigator: Patrick Muck, M.D.
University Hospitals Cleveland Medical Center
11100 Euclid Avenue
Cleveland, OH 44106
Principal Investigator: Karem Harth, M.D.
Pennsylvania
University of Pittsburgh Medical Center (UPMC)
200 Lothrop Street
Pittsburgh, PA 14213
Principal Investigator: Rabih Chaer, M.D.
Rhode Island
The Miriam Hospital
164 Summit Ave.
Providence, RI 02906
Principal Investigator: Peter Soukas, M.D.
Texas
University of Texas Southwestern
5323 Harry Hines Blvd
Dallas, TX 75390
Principal Investigator: Michael Siah, M.D.
Virginia
Sentara Norfolk General Hospital
600 Gresham Dr
Norfolk, VA 23507
Principal Investigator: David Dexter, M.D.
Washington
Lake Washington Vascular Surgeons PLLC
1135 116th Ave NE Suite 305
Bellevue, WA 98004
Principal Investigator: Kathleen Gibson, M.D.
Wisconsin
Medical College of Wisconsin Milwaukee
9200 W Wisconsin Ave
Milwaukee, WI 53226
Principal Investigator: Parag Patel, M.D.
California
Leland Stanford Junior University
300 Pasteur Drive
Stanford, CA 94305
Principal Investigator: Alex Vezeridis, M.D.
Connecticut
Yale University
300 Cedar Street
New Haven, CT 06519
Principal Investigator: Cassius Chaar, M.D.
District of Columbia (D.C.)
MedStar Washington Hospital Center
110 Irving Street Northwest
Washington, DC 20010
Principal Investigator: Steven Abramowitz, M.D.
Illinois
Northwestern University
676 N St. Clair Street, Suite 1400
Chicago, IL 60611
Principal Investigator: Kush Desai, M.D.
Michigan
University of Michigan
Frankel Cardiovascular Center
1425 E Ann St
Ann Arbor, MI 48109
Principal Investigator: Minhaj Khaja, M.D.
New York
Mount Sinai Health System
1 Gustave L. Levy Place
New York, NY 10029
Principal Investigator: Robert Lookstein, M.D.
Weill Cornell Medical College
525 E68th Street
New York, NY 10065
Principal Investigator: Ronald Winokur, M.D.
North Carolina
Atrium Health-Sanger Heart and Vascular Institute
1237 Harding Place, KMOB I, Suite 5100
Charlotte, NC 28204
Principal Investigator: Erin Murphy, M.D.
Ohio
Cleveland Clinic
9500 Euclid Ave
Cleveland, OH 44195
Principal Investigator: Jon Quatromoni, M.D.
OhioHealth Research and Innovation Institute
3705 Olentangy River Road, Suite 100
Columbus, OH 43214
Principal Investigator: Mitch Silver, M.D.
Virginia
Sentara Norfolk General Hospital
600 Gresham Dr
Norfolk, VA 23507
Principal Investigator: David Dexter, M.D.
Washington
Lake Washington Vascular Surgeons PLLC
1135 116th Ave NE Suite 305
Bellevue, WA 98004
Principal Investigator: Kathleen Gibson, M.D.
Australia
Adelaide
Flinders Medical Center
Department of Vascular and Endovascular Surgery
Flinders Drive
Bedford Park, SA 5042S
Australia
Principal Investigator: Phil Puckridge, M.D.
Perth
Sir Charles Gairdner Hospital
Department of Radiology-Intervention
Hospital Ave
Nedlands, WA 6009
Australia
Principal Investigator: Shaun Samuelson, M.D.
Royal Perth Hospital
Wellington Street
Perth, WA 6000
Australia
Principal Investigator: Patrice Mwipatayi, M.D.
England
Cambridge
Box 212, Department of Vascular Surgery
Addenbrooke’s Hospital
Hills Road
Cambridge
CB2 0QQ
UK
Principal Investigator: Manj Gohel, M.D.
London
Guy’s and St Thomas’ NHS Foundation Trust
Academic Department of Surgery
St Thomas’ Hospital
Westminster Bridge Road
London, SE1 7EH
UK
Principal Investigator: Stephen Black, M.D.
Oxford
John Radcliffe - Oxford University Hospitals NHS Foundation Trust
Headley Way, Headington
Oxford
OX3 9DU
UK
Principal Investigator: Emma Watson, M.D.
Germany
Aachen
University of Aachen
Pauwelsstr.30
52074 Aachen
Germany
Principal Investigator: Houman Jalaie, M.D.
Arnsberg
Klinikum Hochsauerland GmbH, Klinik für Angiologie
Stolte Ley 5
Arnsberg 59759
Germany
Principal Investigator: Michael Lichtenberg, M.D.
Ireland
Connaught
University College Hospital GALWAY / Clinical Research Facility Galway
Newcastle Road
Connaught
H91 YR71
Ireland
Principal Investigator: Gerry O’Sullivan, M.D.
Italy
Milan
H San Raffaele
IRCCS Ospedale San Raffaele
Via Olgettina 60
Milan 20132
Italy
Principal Investigator: Domenico Baccellieri, M.D.
Modena
Dept. of Vascular Surgery - International Center of Deep Venous Surgery
Hesperia Hospital
Via Arquà 80/A
Postal Code 41125
Modena, Italy
Principal Investigator: Marzia Lugli, M.D.
New Zealand
Auckland
Auckland City Hospital
2 Park Road Grafton
1023 Auckland
New Zealand
Principal Investigator: Andrew Holden, M.D.
1. Lawrensia S, Khan YS. Inferior Vena Cava Syndrome. In: StatPearls. Treasure Island (FL): StatPearls Publishing; May 20, 2023.
2. The Critical Need for an Iliofemoral Venous Obstruction Classification System. Endovascular Today website. Updated July 2017.
3. Chronic Venous Disease. American Venous Forum website. Updated February 14, 2022. https://www.venousforum.org/patients/what-is-vein-disease/what-is-chronic-venous-disease/
4. Venous Disease. Cleveland Clinic website. Updated February 3, 2023. https://my.clevelandclinic.org/health/diseases/16754-venous-disease
For further information, please refer to the Evaluation of the GORE® VIAFORT Vascular Stent for Treatment of Symptomatic Inferior Vena Cava Obstruction with or without Combined Iliofemoral Obstruction (VNS-21-05) Clinical Study Protocol and Evaluation of the GORE® VIAFORT Vascular Stent for Treatment of Symptomatic Iliofemoral Venous Obstruction (VNS-21-07) Clinical Study Protocol.
There are risks associated with any surgical procedure. Please talk with your doctor about any risks related to these studies. RXOnly
CAUTION — Investigational device. Limited by Federal (or United States) law to investigational use.
Products listed may not be available in all markets
GORE, Together, improving life, VIAFORT and designs are trademarks of W. L. Gore & Associates.
© 2025 W. L. Gore & Associates, Inc.
25PL6011-EN01