CBAS® Heparin Surface
Proven heparin bonding technology for lasting thromboresistance
Dedicated to Innovation: Thromboresistance in Vascular Devices
Gore’s CBAS® Heparin Surface, the proven heparin bonding technology for lasting thromboresistance, is used in many of our interventional and vascular surgery products. End-point covalent bonding keeps heparin anchored to the device, while the bioactive site remains free to interact with the blood to help prevent clotting.*
- Proven heparin availability: Performance-ready heparin active site*,1,4
- Proven heparin bioactivity: Unmatched, persistent ability to take up antithrombin*,2,3
- Proven lasting thromboresistance: Improved surface hemocompatibility resulting from heparin availability and bioactivity*,1-5
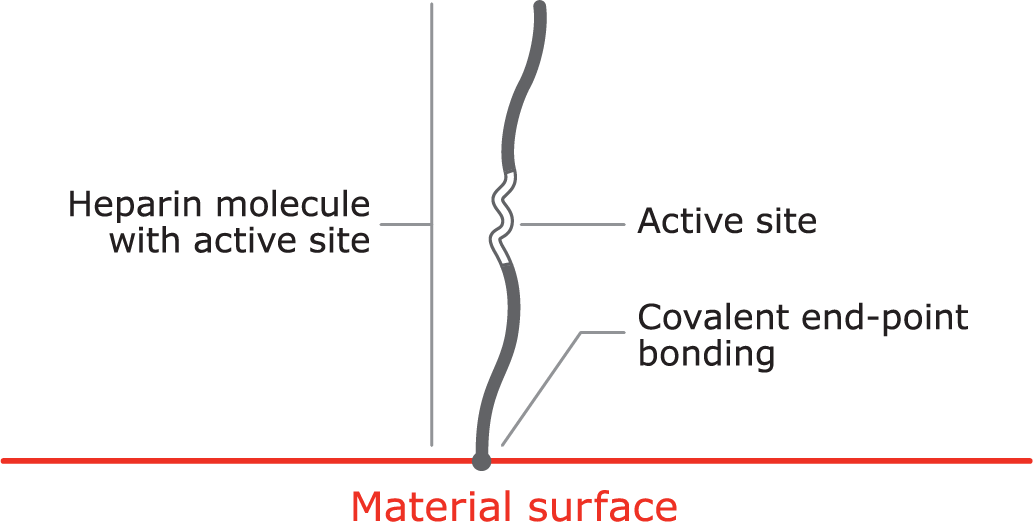
Proprietary covalent end-point bonding
Covalent end-point bonding allows the heparin to extend into the bloodstream, keeping the active site bioavailable, unlike a non-permanent bond that can be washed away in the bloodstream.
- The anticoagulant function of heparin is dependent on the bioavailability of an active site within the molecule.
- Some methods of covalent heparin bonding damage and / or obstruct the active site, and hence destroy heparin’s anticoagulant activity.
- The CBAS® Heparin Surface consists of a proprietary covalent end-point bond that preserves the active site, thus retaining heparin’s anticoagulant activity.
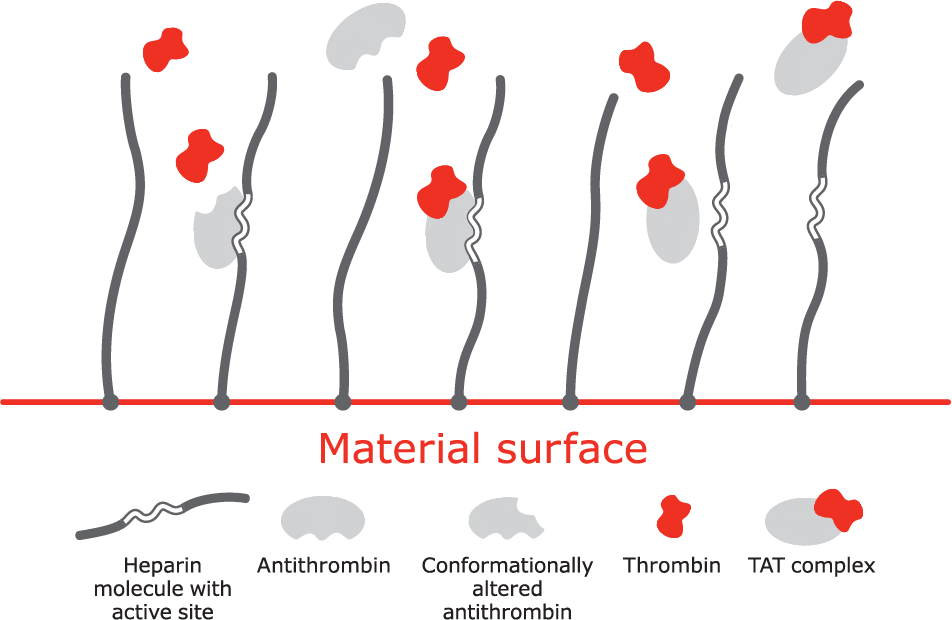
Mechanism of action
A. Bioactive site of the heparin molecule enables antithrombin to bind thrombin.
B. When antithrombin binds to thrombin, a neutral thrombin antithrombin (TAT) complex is formed.
C. Neutral TAT complex detaches from the heparin molecule. Active site becomes available to again bind antithrombin.
- Gore S, Andersson J, Biran R, Underwood C, Riesenfeld J. Heparin surfaces: impact of immobilization chemistry on hemocompatibility and protein adsorption. Journal of Biomedical Materials Research Part B: Applied Biomaterials 2014;102(8):1817-1824.
- Begovac PC, Thomson RC, Fisher JL, Hughson A, Gällhagen A. Improvements in GORE-TEX® Vascular Graft performance by Carmeda® BioActive Surface heparin immobilization. European Journal of Vascular & Endovascular Surgery 2003;25(5):432-437.
- Freeman J, Chen A, Weinberg RJ, Okada T, Chen C, Lin PH. Sustained thromboresistant bioactivity with reduced intimal hyperplasia of heparin-bonded PTFE Propaten Graft in a chronic canine femoral artery bypass model. Annals of Vascular Surgery 2018;49:295-303.
- Biran R, Pond D. Heparin coatings for improving blood compatibility of medical devices. Advanced Drug Delivery Reviews 2017;112:12-23.
- Carmeda AB. Carmeda® BioActive Surface (also known as CBAS® Heparin Surface) Reference List. Upplands Väsby, Sweden: Carmeda AB; 2019. [Reference list]
22589259-EN