
GORE® EXCLUDER® AAA Endoprosthesis
With more than 20 years of experience, the trusted performance of the GORE® EXCLUDER® Device is paired with the intuitive GORE® C3® Delivery System to provide optimal infrarenal seal and reliable results, even in more challenging anatomies.
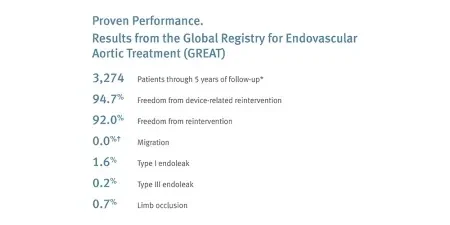
Long-term durability: 96.0% freedom from device-related reintervention and 0.7% limb occlusion through five-year follow-up.*

Twenty years of evidence: Worldwide experience with time-tested results
* GREAT. n = 3,274. To calculate the overall event rates from procedure through end of study period, all subjects who could have had events, regardless of length of follow-up, were included. For outcome data, GREAT only collects site reported serious adverse events. Therefore, all reported endoleaks are defined as serious and require reintervention.
† One peri-procedural migration reported. Zero migrations reported during follow-up through 5 years.
