Endovascular treatment of chronic superficial femoral artery (SFA) occlusion for limb salvage
Challenge
- 83-year-old female with ischemic gangrene of right great toe (Rutherford 6)
- Relevant patient history:
- Diabetes, hypertension, dyslipidemia, obesity, coronary artery disease, atrial fibrillation, chronic kidney disease, chronic obstructive pulmonary disease (COPD)
- Ankle-brachial index (ABI) supra-systemic due to calcified tibial arteries
- Pulse volume recordings demonstrated femoropopliteal occlusive disease
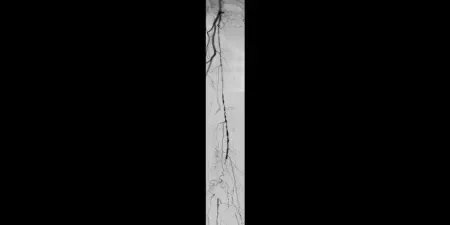
Image: Proximal SFA disease and mid-SFA occlusion
Images courtesy of James Persky, MD. Used with permission.
Procedure
- Contralateral approach to right SFA (March 05, 2013)
- Lesion crossed using straight TERUMO GLIDEWIRE® Guidewire, followed by placement of SPECTRANETICS® QUICK‑CROSS Support Catheter
- Percutaneous transluminal angioplasty (PTA) with 5 mm angioplasty balloon
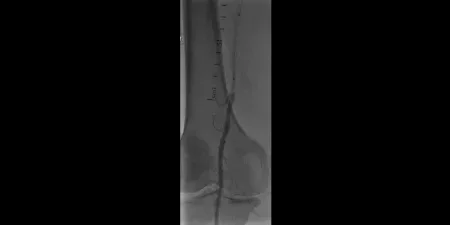
Image: Lesion crossed
Images courtesy of James Persky, MD. Used with permission.
Result
- Proximal device placed successfully at the ostium of the SFA
- Correct sizing with 5 mm device
- Excellent radiographic result
- Patient’s ulcer healed after intervention
- Stent grafts patent through most recent follow-up in late 2014
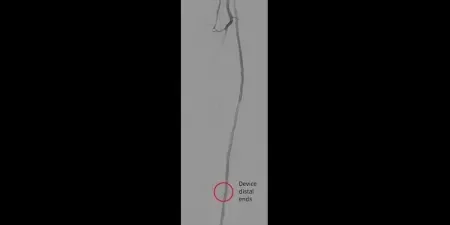
Image: Post-placement of three 5 mm GORE® VIABAHN® Devices
Images courtesy of James Persky, MD. Used with permission.
Case takeaways
- Limb salvage by endoluminal bypass in Rutherford 6 limb
- While initial PTA demonstrated a good result, diabetic patients have diffuse disease and the arteriogram understates the plaque burden in these patients
- Cover healthy-to-healthy to the extent possible
We opt for greater stent coverage beyond the segment of disease.
We also employ oblique views of the take-off of the SFA so we can treat the diseased artery at the take-off of the SFA without compromising flow in the profunda femoris artery.— James Persky, MD, Cleveland, Ohio
TERUMO and GLIDEWIRE® are trademarks of Terumo Medical Corporation.
SPECTRANETICS® and QUICK CROSS are trademarks of Spectranetics Corporation.
The outcomes and observations reported are based on individual case experience and the patients treated. The steps described here may not be complete, and are not intended to be a replacement for the Instructions for Use or the education, training and professional judgment of Healthcare Providers. Healthcare Providers remain solely responsible for making decisions about patient care and the use of medical technologies.