Advancing endovascular options: New endovascular devices and insights to extend aortic treatment to more patients
ADVANCING ENDOVASCULAR OPTIONS
Sponsored by Gore & Associates
Precision and accuracy in the distal landing zone
A case report demonstrating the precise deployment qualities of the GORE® TAG® Conformable Thoracic Stent Graft with ACTIVE CONTROL System.
Hence J.M. Verhagen, MD, PhD
Sander ten Raa, MD, PhD
Marie Josee van Rijn, MD, PhD
Stent graft deployment systems for thoracic endovascular aneurysm repair (TEVAR) are often designed with a focus on proximal landing accuracy due to the common challenges associated with implantations in arch anatomies and with landing zones near the supra-aortic branch vessels. However, precision of the distal graft deployment is also critical to good clinical outcomes, especially in patients whose aortic disease extends near the visceral arteries. Precise deployments in such distal anatomies can be challenging because of the tendency for the stent graft to move upward in the aorta, which often occurs as the device expands off of the catheter and finds apposition with the aortic vessel wall. As devices adapt to the surrounding anatomy, their positioning may deviate from the path taken by the guidewire and catheter, resulting in upward movement from the intended target location.
New developments in TEVAR deployment systems enable device control and deployment precision in distal as well as proximal target landing zones. In the following case, we present an aneurysm patient who was successfully treated using the GORE® TAG® Conformable Thoracic Stent Graft with ACTIVE CONTROL System.*
CASE REPORT
A 74-year-old man was transferred from an outside hospital to our center with complaints of a sudden onset of severe pain between the shoulder blades. He was hemodynamically stable with a systolic blood pressure of > 180 mm Hg. An urgently performed CT scan including very late–phase imaging revealed an intramural hematoma (IMH) extending from the origin of the left subclavian artery (LSA) to the level of the celiac trunk without any further complications.
His past medical history included stage 3 chronic obstructive pulmonary disease, hypertension, and an open aortic repair with a bifurcated graft for a large abdominal aortic aneurysm (AAA) 6 years earlier. Furthermore, he was known to have a thoracic aortic aneurysm (TAA) that was approximately 57 mm in diameter, which was closely monitored over the recent years and showed minimal growth.
The patient was treated with continuous ß-blocker infusion (labetalol) with rapid normalization of blood pressure to approximately 120 mm Hg systolic and relief of all pain symptoms. An urgent TEVAR was considered but due to the lack of healthy proximal (Figure 1) and distal sealing zones, the presumed high risk of paraplegia, as well as his favorable response to antihypertensive treatment, a conservative approach was chosen.
Images on a repeat CTA after 10 days were unchanged, and the patient was discharged in good condition. Further CTA imaging 1 month after presentation showed no further aortic dilatation and clear resolution of the thickness and extent of the IMH. Further optimization of medical treatment of cardiovascular risk factors was established during outpatient visits.
Six months after onset of his IMH, he returned for a scheduled follow-up visit including new imaging that showed almost complete resolution of the IMH (Figure 2) and a slight increase in maximum diameter to 59 mm. He was discussed in our aortic group again, and the decision was made to continue following a conservative strategy up to a maximum diameter of > 60 mm, as his risk of paraplegia was presumed to be far above average due to the extensive coverage of the thoracic aorta by TEVAR, in addition to his former open AAA repair.
CTA 6 months later showed further dilatation of the TAA to 63 mm, and the decision was made to offer minimally invasive treatment by TEVAR. Due to his specific anatomy, a graft with optimal deployment precision was necessary, especially distally, as deployment of the graft close to the origin of the celiac trunk in order to maximize the distal sealing zone was presumed essential. For this purpose, the new GORE TAG Conformable Thoracic Stent Graft with ACTIVE CONTROL System seemed to be the ideal device.
First, a spinal drain was inserted for neuroprotection, after which the patient was placed under general anesthesia. Through open access, a 24 Fr GORE® DrySeal Flex Introducer Sheath was placed through the right common femoral artery. The proximal GORE TAG Conformable Device (40 mm diameter, 200 mm length; with 15%–20% oversizing) was inserted up to the level of the distal arch, and a pigtail catheter was placed into the ascending aorta through the same sheath. After digital subtraction angiography (DSA) with the image intensifier at 45° left anterior oblique, the proximal device was deployed, aiming just distal to the LSA (length of proximal landing, 35 mm). The GORE TAG Conformable Device deployed exactly at the intended position, with no change in position during the different deployment steps (Figure 3).
The distal device (40 mm diameter, 200 mm length) was then inserted, and DSA was performed with the image intensifier at 50° right anterior oblique for optimal visualization of the origin of the celiac trunk. Deployment followed the intuitive steps, and again, perfect placement of the GORE TAG Conformable Device was achieved, this time aiming the distal part of the stent graft in order to be as close as possible to the celiac trunk (Figure 4; Videos 1 and 2). The intervention lasted < 90 minutes, and the patient recovered uneventfully with full neurologic function. Postoperative CTA showed complete exclusion of the TAA with optimal coverage of available proximal and distal landing zones (Figure 5).
DISCUSSION
The GORE TAG Conformable Thoracic Stent Graft with ACTIVE CONTROL System includes several unique features that contributed to the successful treatment of this patient. First, the nested handle design of the deployment system remained very easy to use despite the increased number of steps for staged deployment and angulation. Given our success with the straightforward deployment of the existing Conformable GORE® TAG® Thoracic Endoprosthesis, we wondered whether the added steps and functionality of the new device would complicate the deployment process, as more steps would have to be remembered. However, we found the operative steps to be exceptionally easy and intuitive with absolute ease of mind during the procedure.

Figure 1. CTA image of the distal aortic arch at the level of the LSA. A clear, thick layer of IMH is seen, excluding a healthy proximal landing zone.

Figure 2. CTA image of the distal aortic arch at the level of the LSA. The thick IMH has almost completely vanished, resulting in a relatively healthy proximal landing zone.

Figure 3. Deployment of the proximal device at intermediate diameter with continuous blood flow (A) and at full diameter (B).

Figure 4. Accurate placement of the distal device at the level of the celiac trunk.
Angiography of the distal device at its intermediate diameter. Staged deployment to intermediate diameter allows for visualization, and if necessary, refinement of the stent graft position. At this stage, there is no vessel wall apposition and continuous blood flow is ensured.
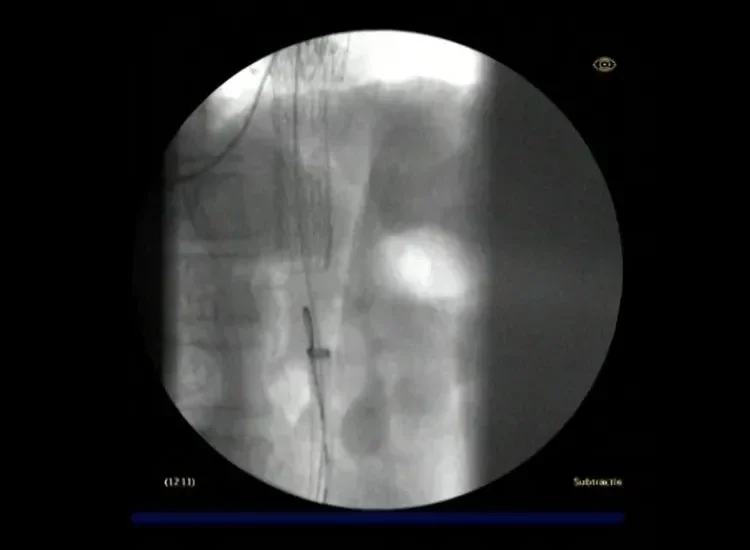
Angiography of the distal device during secondary deployment to full diameter. The device opens from trailing to leading end during this step, thus allowing for vessel wall apposition and passive fixation on the distal end before the rest of the device is opened. A lock wire keeps the stent graft secured to the catheter throughout the deployment to enable control and stability throughout the procedure.

Figure 5. Postoperative CTA demonstrating successful exclusion of the aneurysm with optimal device placement at both proximal and distal landing zones.
Second, the device was very stable during the deployment process. We were able to achieve perfect positioning of the first stent graft in the aortic arch just distal to the LSA without any movement during deployment. We were pleasantly surprised that no windsock effect was noted, likely due to the primary deployment to intermediate diameter, allowing continuous blood flow through the graft during the entire deployment process. This lack of windsock effect was observed again during the distal device placement as well.
Last, and most importantly for this case, the new deployment system enabled very precise and predictable distal device placement. Most thoracic stent grafts show brisk and clear upward movement of the distal device during the last stages of deployment when placed at the level of the celiac trunk, making it difficult to land the graft exactly at the intended position. In this case, exact positioning of the distal device was key in order to achieve sufficient sealing length. This worked perfectly with the GORE TAG Conformable Thoracic Stent Graft due to the hemodynamic stability of the staged deployment but also the lock wire that ensured that the device remained fixed onto the catheter throughout the deployment process.
Staged deployment to intermediate diameter provides an opportunity to refine device placement during deployment, but because we did not observe any changes in device position during the different deployment stages and were pleased with the precision with which the device landed at the target, we did not need to make any adjustments. Furthermore, because secondary deployment to full diameter is designed to open the device from the trailing to leading end, this allowed us to visualize and control precise distal positioning of the device and to gain full vessel wall apposition just proximal to the celiac trunk before completing this deployment step.
CONCLUSION
The GORE TAG Conformable Thoracic Stent Graft with ACTIVE CONTROL System includes new design features like staged deployment and lock wire fixation of the device to the catheter that enhances control and precision throughout the deployment process. These advancements are beneficial in ensuring accurate device placement and maximizing seal length in anatomies where the distal, as well as proximal, landing zone is critical. Importantly, the system remains easy to use and the handle operation is intuitive.
Products listed may not be available in all markets.
See all products by region:
Hence J.M. Verhagen, MD, PhD
Professor and Chief of Vascular Surgery
Erasmus University Medical Center
Rotterdam, the Netherlands
Disclosures: Paid consultant to Medtronic, Gore & Associates, Endologix, Inc., and Arsenal AAA, LLC.
Sander ten Raa, MD, PhD
Department of Vascular Surgery
Erasmus University Medical Centre
Rotterdam, the Netherlands
Disclosures: None.
Marie Josee van Rijn, MD, PhD
Department of Vascular Surgery
Erasmus University Medical Centre
Rotterdam, the Netherlands
Disclosures: None.
